Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.molmet.2020.01.004 Mélissa Léveillé 1 , Aurèle Besse-Patin 1 , Nathalie Jouvet 2 , Aysim Gunes 2 , Sarah Sczelecki 2 , Stewart Jeromson 3 , Naveen P Khan 2 , Cindy Baldwin 3 , Annie Dumouchel 3 , Jorge C Correia 4 , Paulo R Jannig 4 , Jonathan Boulais 3 , Jorge L Ruas 4 , Jennifer L Estall 5
|
Objective
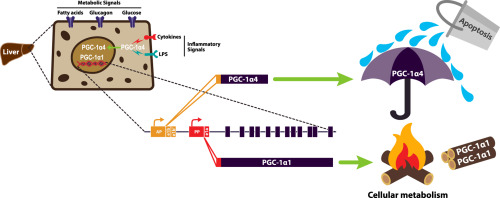
The liver is regularly exposed to changing metabolic and inflammatory environments. It must sense and adapt to metabolic need while balancing resources required to protect itself from insult. Peroxisome proliferator activated receptor gamma coactivator-1 alpha (PGC-1α) is a transcriptional coactivator expressed as multiple, alternatively spliced variants transcribed from different promoters that coordinate metabolic adaptation and protect against inflammation. It is not known how PGC-1α integrates extracellular signals to balance metabolic and anti-inflammatory outcomes.
Methods
Primary mouse hepatocytes were used to evaluate the role(s) of different PGC-1α proteins in regulating hepatic metabolism and inflammatory signaling downstream of tumor necrosis factor alpha (TNFα). Gene expression and signaling analysis were combined with biochemical measurement of apoptosis using gain- and loss-of-function in vitro and in vivo.
Results
Hepatocytes expressed multiple isoforms of PGC-1α, including PGC-1α4, which microarray analysis showed had common and isoform-specific functions linked to metabolism and inflammation compared with canonical PGC-1α1. Whereas PGC-1α1 primarily impacted gene programs of nutrient metabolism and mitochondrial biology, TNFα signaling showed several pathways related to innate immunity and cell death downstream of PGC-1α4. Gain- and loss-of-function models illustrated that PGC-1α4 uniquely enhanced expression of anti-apoptotic gene programs and attenuated hepatocyte apoptosis in response to TNFα or lipopolysaccharide (LPS). This was in contrast to PGC-1α1, which decreased the expression of a wide inflammatory gene network but did not prevent hepatocyte death in response to cytokines.
Conclusions
PGC-1α variants have distinct, yet complementary roles in hepatic responses to metabolism and inflammation, and we identify PGC-1α4 as an important mitigator of apoptosis.
中文翻译:

PGC-1α亚型可协调平衡炎症环境中的肝脏代谢和细胞凋亡。
目的
肝脏定期暴露于变化的代谢和炎性环境。它必须感知并适应新陈代谢的需要,同时平衡保护自己免受侵害所需的资源。过氧化物酶体增殖物激活的受体γcoactivator-1 alpha(PGC-1α)是一种转录coactivator,表达为多种,可变剪接的变体,由不同的启动子转录而成,可协调代谢适应并保护炎症。未知PGC-1α如何整合细胞外信号以平衡代谢和抗炎结局。
方法
原代小鼠肝细胞用于评估不同PGC-1α蛋白在调节肿瘤坏死因子α(TNFα)下游的肝代谢和炎症信号中的作用。基因表达和信令分析用使用获得和丧失功能的细胞凋亡的生化测定结合在体外和体内。
结果
肝细胞表达了包括PGC-1α4在内的多种PGC-1α亚型,与常规的PGC-1α1相比,微阵列分析显示它们具有与代谢和炎症相关的常见和亚型特异性功能。PGC-1α1主要影响营养代谢和线粒体生物学的基因程序,而TNFα信号传导则显示了与PGC-1α4下游的先天免疫和细胞死亡相关的几种途径。功能获得和丧失功能模型表明,PGC-1α4独特地增强了抗凋亡基因程序的表达,并减弱了对TNFα或脂多糖(LPS)的肝细胞凋亡。这与PGC-1α1相反,PGC-1α1降低了广泛的炎性基因网络的表达,但并未阻止肝细胞因细胞因子而死亡。
结论
PGC-1α变体在肝脏对代谢和炎症的反应中具有独特但互补的作用,我们将PGC-1α4鉴定为凋亡的重要缓解剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号