当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring Chloride Selectivity and Halogenase Regioselectivity of the SalL Enzyme through Quantum Mechanical/Molecular Mechanical Modeling.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jcim.9b01079 Paulo R M Pereira 1 , Jéssica de O Araújo 1 , José Rogério A Silva 1 , Cláudio N Alves 1 , Jerônimo Lameira 1, 2 , Anderson H Lima 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jcim.9b01079 Paulo R M Pereira 1 , Jéssica de O Araújo 1 , José Rogério A Silva 1 , Cláudio N Alves 1 , Jerônimo Lameira 1, 2 , Anderson H Lima 1
Affiliation
|
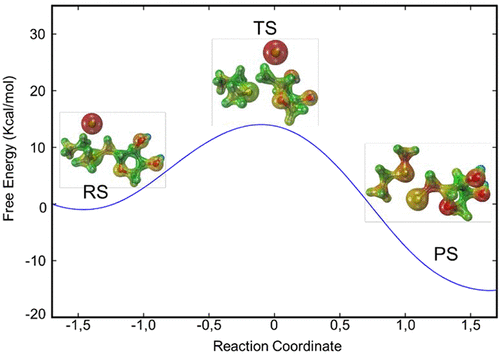
The catalytic mechanism of SalL chlorinase has been investigated by combining quantum mechanical/molecular mechanical (QM/MM) techniques and umbrella sampling simulations to compute free energy profiles. Our results shed light on the interesting fact that the substitution of chloride with fluorine in SalL chlorinase leads to a loss of halogenase activity. The potential of mean force based on DFTB3/MM analysis shows that fluorination corresponds to a barrier 13.5 kcal·mol-1 higher than chlorination. Additionally, our results present a molecular description of SalL acting as a chlorinase instead of a methyl-halide transferase.
中文翻译:

通过量子力学/分子力学建模探索SalL酶的氯化物选择性和卤化酶区域选择性。
SalL氯化酶的催化机理已通过结合量子力学/分子力学(QM / MM)技术和伞式采样模拟来计算自由能分布图。我们的结果揭示了一个有趣的事实,那就是SalL氯化酶中的氟取代氯会导致卤化酶活性降低。基于DFTB3 / MM分析的平均力潜力显示,氟化对应的屏障比氯化高13.5 kcal·mol-1。此外,我们的结果提供了作为氯化酶而不是甲基卤化物转移酶的SalL的分子描述。
更新日期:2020-01-24
中文翻译:

通过量子力学/分子力学建模探索SalL酶的氯化物选择性和卤化酶区域选择性。
SalL氯化酶的催化机理已通过结合量子力学/分子力学(QM / MM)技术和伞式采样模拟来计算自由能分布图。我们的结果揭示了一个有趣的事实,那就是SalL氯化酶中的氟取代氯会导致卤化酶活性降低。基于DFTB3 / MM分析的平均力潜力显示,氟化对应的屏障比氯化高13.5 kcal·mol-1。此外,我们的结果提供了作为氯化酶而不是甲基卤化物转移酶的SalL的分子描述。









































 京公网安备 11010802027423号
京公网安备 11010802027423号