当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the Overlooked Critical Role of the pH Value on the Kinetics of the 4-Nitrophenol NaBH4-Reduction Catalyzed by Noble-Metal Nanoparticles (Pt, Pd, and Au)
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b07114 Roland Grzeschik 1 , Daniel Schäfer 1 , Tim Holtum 1 , Sebastian Küpper 1 , Axel Hoffmann 1 , Sebastian Schlücker 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b07114 Roland Grzeschik 1 , Daniel Schäfer 1 , Tim Holtum 1 , Sebastian Küpper 1 , Axel Hoffmann 1 , Sebastian Schlücker 1
Affiliation
|
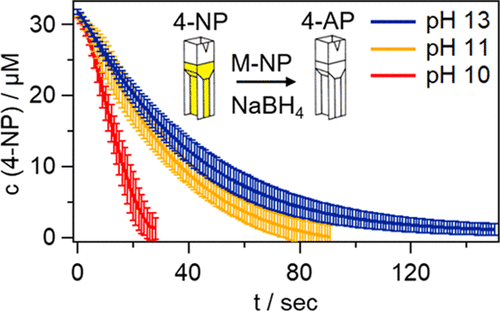
The reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) with sodium borohydride (NaBH4) in aqueous media is by far the most widely used reaction for testing the catalytic performance of a large variety of metal nanoparticles (MNPs) using UV/vis absorption spectroscopy. Huge differences in the kinetic rate constant of the catalytic 4-NP to 4-AP conversion as a function of material composition, size, and morphology have been reported by many different groups; so far, these have been exclusively attributed to the catalytic activity of the corresponding MNPs. In this study, we study the overlooked critical role of the pH value on the kinetics of this important reaction. We observe a strong pH dependence of both rate constant and reaction order, which we attribute to the pH-dependent hydrolysis of the reducing agent NaBH4 in water. In each hydrolysis step, molecular hydrogen as a second reducing agent is produced. Overall, two competing pathways result in: slow hydride versus fast hydrogen reduction. Kinetic simulations based on a model including all relevant species are capable of quantitatively describing all experimental results for three different noble-metal nanoparticle catalysts (Au, Pt, and Pd). Most importantly, for a fair interlaboratory comparison of the catalytic performances of MNPs, we recommend to report rate constants of the 4-NP to 4-AP conversion at pH 13 by strict pH control using a strong base such as NaOH since at pH 13 the hydrolysis of NaBH4 in aqueous solution is slowed down significantly.
中文翻译:

贵金属纳米粒子(Pt,Pd和Au)催化pH值对4-硝基苯酚NaBH 4还原动力学的关键作用
硼氢化钠(NaBH 4将4-硝基苯酚(4-NP)还原为4-氨基苯酚(4-AP)迄今为止,水性介质是最广泛使用的反应,用于使用紫外/可见吸收光谱法测试多种金属纳米颗粒(MNP)的催化性能。许多不同的研究小组已经报道了催化4-NP转化为4-AP的动力学速率常数与材料组成,尺寸和形态有关的巨大差异。迄今为止,这些仅归因于相应MNP的催化活性。在这项研究中,我们研究了pH值对这一重要反应动力学的重要作用。我们观察到速率常数和反应顺序都对pH有很强的依赖性,这归因于还原剂NaBH 4的pH依赖性水解在水里。在每个水解步骤中,产生分子氢作为第二还原剂。总体而言,两种竞争途径导致:氢化物缓慢还原与氢快速还原。基于包括所有相关物种的模型的动力学模拟能够定量描述三种不同的贵金属纳米颗粒催化剂(Au,Pt和Pd)的所有实验结果。最重要的是,为了对MNP的催化性能进行公平的实验室间比较,我们建议报告使用强碱(如NaOH)通过严格的pH控制在pH 13时4-NP转化为4-AP的速率常数,因为在pH 13时NaBH 4在水溶液中的水解显着减慢。
更新日期:2020-01-26
中文翻译:

贵金属纳米粒子(Pt,Pd和Au)催化pH值对4-硝基苯酚NaBH 4还原动力学的关键作用
硼氢化钠(NaBH 4将4-硝基苯酚(4-NP)还原为4-氨基苯酚(4-AP)迄今为止,水性介质是最广泛使用的反应,用于使用紫外/可见吸收光谱法测试多种金属纳米颗粒(MNP)的催化性能。许多不同的研究小组已经报道了催化4-NP转化为4-AP的动力学速率常数与材料组成,尺寸和形态有关的巨大差异。迄今为止,这些仅归因于相应MNP的催化活性。在这项研究中,我们研究了pH值对这一重要反应动力学的重要作用。我们观察到速率常数和反应顺序都对pH有很强的依赖性,这归因于还原剂NaBH 4的pH依赖性水解在水里。在每个水解步骤中,产生分子氢作为第二还原剂。总体而言,两种竞争途径导致:氢化物缓慢还原与氢快速还原。基于包括所有相关物种的模型的动力学模拟能够定量描述三种不同的贵金属纳米颗粒催化剂(Au,Pt和Pd)的所有实验结果。最重要的是,为了对MNP的催化性能进行公平的实验室间比较,我们建议报告使用强碱(如NaOH)通过严格的pH控制在pH 13时4-NP转化为4-AP的速率常数,因为在pH 13时NaBH 4在水溶液中的水解显着减慢。



























 京公网安备 11010802027423号
京公网安备 11010802027423号