当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoelectron Spectroscopy on Polycyclic Hydrocarbon–F6TCNNQ Interfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b08409 Martin Hantusch 1 , Robert Kuhrt 1 , Martin Knupfer 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b08409 Martin Hantusch 1 , Robert Kuhrt 1 , Martin Knupfer 1
Affiliation
|
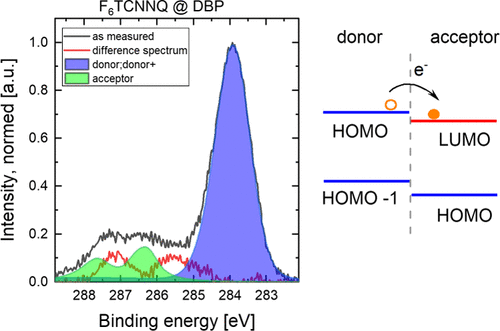
The organic heterojunction between polycyclic hydrocarbons [pentacene, dibenzo[a,l]pentacene (DBP)] and hexafluorotetracyanonaphthoquinodimethane (F6TCNNQ) was investigated by photoelectron spectroscopy. The heterojunction consisted of thin films prepared by a stepwise evaporation process. As a result, a charge-transfer interface is formed, where both polycyclic hydrocarbons acted as electron donors and F6TCNNQ acted as an electron acceptor. This charge transfer was determined as a localized charge transfer with an ionic character because the F6TCNNQ anion was observed in the characteristic nitrogen and carbon core levels and the valence-level data showed insulating/semiconducting behavior. The additional benzene rings in DBP lead to a stronger pronunciation of the F6TCNNQ anion characteristics in the heterojunction compared to that in the pentacene heterojunction because of a different interface morphology.
中文翻译:

多环烃–F 6 TCNNQ接口上的光电子能谱
通过光电子能谱研究了多环烃[并五苯,二苯并[ a,l ]并五苯(DBP)]和六氟四氰基萘并邻醌二甲烷(F 6 TCNNQ)之间的有机杂结。异质结由通过逐步蒸发工艺制备的薄膜组成。结果,形成电荷转移界面,其中两个多环烃都充当电子给体,而F 6 TCNNQ充当电子受体。该电荷转移被确定为具有离子特性的局部电荷转移,因为F 6在特征氮和碳核能级中观察到TCNNQ阴离子,化合价级数据显示出绝缘/半导体行为。DBP中额外的苯环与并五苯异质结中的F 6 TCNNQ阴离子特征相比,由于界面形态不同,导致其F 6 TCNNQ阴离子特性的发音更强。
更新日期:2020-01-23
中文翻译:

多环烃–F 6 TCNNQ接口上的光电子能谱
通过光电子能谱研究了多环烃[并五苯,二苯并[ a,l ]并五苯(DBP)]和六氟四氰基萘并邻醌二甲烷(F 6 TCNNQ)之间的有机杂结。异质结由通过逐步蒸发工艺制备的薄膜组成。结果,形成电荷转移界面,其中两个多环烃都充当电子给体,而F 6 TCNNQ充当电子受体。该电荷转移被确定为具有离子特性的局部电荷转移,因为F 6在特征氮和碳核能级中观察到TCNNQ阴离子,化合价级数据显示出绝缘/半导体行为。DBP中额外的苯环与并五苯异质结中的F 6 TCNNQ阴离子特征相比,由于界面形态不同,导致其F 6 TCNNQ阴离子特性的发音更强。











































 京公网安备 11010802027423号
京公网安备 11010802027423号