当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple drug binding modes in Mycobacterium tuberculosis CYP51B1.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jinorgbio.2020.110994 Molly M Lockart 1 , Joseph T Butler 1 , Carson J Mize 1 , Morgan N Fair 1 , Alex A Cruce 1 , Kip P Conner 2 , William M Atkins 2 , Michael K Bowman 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jinorgbio.2020.110994 Molly M Lockart 1 , Joseph T Butler 1 , Carson J Mize 1 , Morgan N Fair 1 , Alex A Cruce 1 , Kip P Conner 2 , William M Atkins 2 , Michael K Bowman 1
Affiliation
|
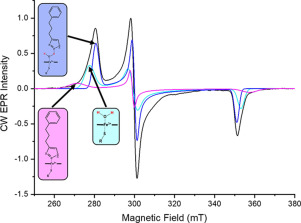
The Mycobacterium tuberculosis (Mtb) genome encodes 20 different cytochrome P450 enzymes (CYPs), many of which serve essential biosynthetic roles. CYP51B1, the Mtb version of eukaryotic sterol demethylase, remains a potential therapeutic target. The binding of three drug fragments containing nitrogen heterocycles to CYP51B1 is studied here by continuous wave (CW) and pulsed electron paramagnetic resonance (EPR) techniques to determine how each drug fragment binds to the heme active-site. All three drug fragments form a mixture of complexes, some of which retain the axial water ligand from the resting state. Hyperfine sublevel correlation spectroscopy (HYSCORE) and electron-nuclear double resonance spectroscopy (ENDOR) observe protons of the axial water and on the drug fragments that reveal drug binding modes. Binding in CYP51B1 is complicated by the presence of multiple binding modes that coexist in the same solution. These results aid our understanding of CYP-inhibitor interactions and will help guide future inhibitor design.
更新日期:2020-01-13











































 京公网安备 11010802027423号
京公网安备 11010802027423号