当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Towards the control of the biological identity of nanobiomaterials: Impact of the structure of 011¯0 surface terminations of nanohydroxyapatite on the conformation of adsorbed proteins.
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.colsurfb.2020.110780 Federico Catalano 1 , Pavlo Ivanchenko 1 , Erica Rebba 1 , Yuriy Sakhno 1 , Gabriele Alberto 1 , Galyna Dovbeshko 2 , Gianmario Martra 1
Colloids and Surfaces B: Biointerfaces ( IF 5.8 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.colsurfb.2020.110780 Federico Catalano 1 , Pavlo Ivanchenko 1 , Erica Rebba 1 , Yuriy Sakhno 1 , Gabriele Alberto 1 , Galyna Dovbeshko 2 , Gianmario Martra 1
Affiliation
|
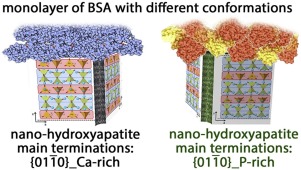
High-resolution transmission electron microscopy, ζ-potential and in-situ IR spectroscopy of adsorbed CO were combined for elucidating the ratio between {011¯0}_ Ca-rich: {011¯0}_ P-rich terminations of {011¯0} facets, i.e. the surfaces with the highest morphological importance, in two nanohydroxyapatite samples. Bovine serum albumin was found to form at least a monolayer on the surface left accessible to protein molecules by the agglomeration of nanoparticles when suspended in the buffered incubation medium. Noticeably, the conformation of adsorbed proteins appeared sensitive to the ratio between the two types of {011¯0} terminations, also resulting in a difference in the surface exposed toward the exterior by the adsorbed protein layer(s).
中文翻译:

努力控制纳米生物材料的生物特性:纳米羟基磷灰石的011’0表面终止结构对吸附蛋白质构象的影响。
结合高分辨率透射电子显微镜,ζ电位和吸附式CO的原位红外光谱,以阐明{011’}富Ca的{011’0} _富P终端之间的比率。 0}刻面,即在两个纳米羟基磷灰石样品中具有最高形态重要性的表面。发现牛血清白蛋白悬浮在缓冲温育培养基中时,由于纳米颗粒的团聚,在蛋白质分子易于接近的表面上形成至少一个单层。明显地,吸附的蛋白质的构象似乎对两种类型的{011’0}末端之间的比率敏感,这也导致被吸附的蛋白质层暴露于外部的表面不同。
更新日期:2020-01-11
中文翻译:

努力控制纳米生物材料的生物特性:纳米羟基磷灰石的011’0表面终止结构对吸附蛋白质构象的影响。
结合高分辨率透射电子显微镜,ζ电位和吸附式CO的原位红外光谱,以阐明{011’}富Ca的{011’0} _富P终端之间的比率。 0}刻面,即在两个纳米羟基磷灰石样品中具有最高形态重要性的表面。发现牛血清白蛋白悬浮在缓冲温育培养基中时,由于纳米颗粒的团聚,在蛋白质分子易于接近的表面上形成至少一个单层。明显地,吸附的蛋白质的构象似乎对两种类型的{011’0}末端之间的比率敏感,这也导致被吸附的蛋白质层暴露于外部的表面不同。



























 京公网安备 11010802027423号
京公网安备 11010802027423号