当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogenic K-Ras4B Dimerization Enhances Downstream Mitogen-activated Protein Kinase Signaling.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jmb.2020.01.002 Serena Muratcioglu 1 , Cihan Aydin 1 , Ezgi Odabasi 2 , E Sila Ozdemir 1 , Elif Nur Firat-Karalar 2 , Hyunbum Jang 3 , Chung-Jung Tsai 3 , Ruth Nussinov 4 , Ibrahim Halil Kavakli 5 , Attila Gursoy 6 , Ozlem Keskin 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jmb.2020.01.002 Serena Muratcioglu 1 , Cihan Aydin 1 , Ezgi Odabasi 2 , E Sila Ozdemir 1 , Elif Nur Firat-Karalar 2 , Hyunbum Jang 3 , Chung-Jung Tsai 3 , Ruth Nussinov 4 , Ibrahim Halil Kavakli 5 , Attila Gursoy 6 , Ozlem Keskin 1
Affiliation
|
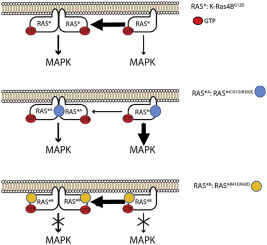
Ras recruits and activates effectors that transmit receptor-initiated signals. Monomeric Ras can bind Raf; however, Raf's activation requires dimerization, which can be facilitated by Ras dimerization. Previously, we showed that active K-Ras4B dimerizes in silico and in vitro through two major interfaces: (i) β-interface, mapped to Switch I and effector-binding regions, (ii) α-interface at the allosteric lobe. Here, we chose constitutively active K-Ras4B as our control and two double mutants (K101D and R102E; and R41E and K42D) in the α- and β-interfaces. Two of the mutations are from The Cancer Genome Atlas (TCGA) and the Catalogue Of Somatic Mutations In Cancer (COSMIC) data sets. R41 and R102 are found in several adenocarcinomas in Ras isoforms. We performed site-directed mutagenesis, cellular localization experiments, and molecular dynamics (MD) simulations to assess the impact of the mutations on K-Ras4B dimerization and function. α-interface K101D/R102E double mutations reduced dimerization but only slightly reduced downstream phosphorylated extracellular signal-regulated kinase (ERK) (pERK) levels. While β-interface R41E/K42D double mutations did not interfere with dimerization, they almost completely blocked K-Ras4B-mediated ERK phosphorylation. Both double mutations increased downstream phosphorylated Akt (pAkt) levels in cells. Changes in pERK and pAkt levels altered ERK- and Akt-regulated gene expressions, such as EGR1, JUN, and BCL2L11. These results underscore the role of the α-interface in K-Ras4B homodimerization and the β-surface in effector binding. MD simulations highlight that the membrane and hypervariable region (HVR) interact with both α- and β-interfaces of K-Ras4B mutants, respectively, inhibiting homodimerization and probably effector binding. Mutations at both interfaces interfered with mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase signaling but in different forms and extents. We conclude that dimerization is not necessary but enhances downstream MAPK signaling.
中文翻译:

致癌 K-Ras4B 二聚化增强下游丝裂原激活蛋白激酶信号传导。
Ras 招募并激活传递受体启动信号的效应器。单体Ras可以结合Raf;然而,Raf 的激活需要二聚化,而 Ras 二聚化可以促进二聚化。之前,我们证明活性 K-Ras4B 在计算机和体外通过两个主要界面二聚化:(i)映射到 Switch I 和效应器结合区域的 β 界面,(ii)变构叶的 α 界面。在这里,我们选择组成型活性 K-Ras4B 作为对照,并选择 α-和 β-界面中的两个双突变体(K101D 和 R102E;以及 R41E 和 K42D)。其中两个突变来自癌症基因组图谱 (TCGA) 和癌症体细胞突变目录 (COSMIC) 数据集。 R41 和 R102 存在于 Ras 亚型的多种腺癌中。我们进行了定点诱变、细胞定位实验和分子动力学 (MD) 模拟,以评估突变对 K-Ras4B 二聚化和功能的影响。 α-界面 K101D/R102E 双突变减少了二聚化,但仅略微降低了下游磷酸化细胞外信号调节激酶 (ERK) (pERK) 水平。虽然 β 界面 R41E/K42D 双突变不会干扰二聚化,但它们几乎完全阻断 K-Ras4B 介导的 ERK 磷酸化。两种双突变均增加了细胞中下游磷酸化 Akt (pAkt) 水平。 pERK 和 pAkt 水平的变化改变了 ERK 和 Akt 调节的基因表达,例如 EGR1、JUN 和 BCL2L11。这些结果强调了 α-界面在 K-Ras4B 同二聚化中的作用以及 β-表面在效应子结合中的作用。 MD 模拟强调膜和高变区 (HVR) 分别与 K-Ras4B 突变体的 α 和 β 界面相互作用,抑制同源二聚化并可能抑制效应子结合。 两个界面的突变都会干扰丝裂原激活蛋白激酶 (MAPK) 和磷酸肌醇 3 激酶信号传导,但形式和程度不同。我们得出结论,二聚化不是必需的,但可以增强下游 MAPK 信号传导。
更新日期:2020-01-11
中文翻译:

致癌 K-Ras4B 二聚化增强下游丝裂原激活蛋白激酶信号传导。
Ras 招募并激活传递受体启动信号的效应器。单体Ras可以结合Raf;然而,Raf 的激活需要二聚化,而 Ras 二聚化可以促进二聚化。之前,我们证明活性 K-Ras4B 在计算机和体外通过两个主要界面二聚化:(i)映射到 Switch I 和效应器结合区域的 β 界面,(ii)变构叶的 α 界面。在这里,我们选择组成型活性 K-Ras4B 作为对照,并选择 α-和 β-界面中的两个双突变体(K101D 和 R102E;以及 R41E 和 K42D)。其中两个突变来自癌症基因组图谱 (TCGA) 和癌症体细胞突变目录 (COSMIC) 数据集。 R41 和 R102 存在于 Ras 亚型的多种腺癌中。我们进行了定点诱变、细胞定位实验和分子动力学 (MD) 模拟,以评估突变对 K-Ras4B 二聚化和功能的影响。 α-界面 K101D/R102E 双突变减少了二聚化,但仅略微降低了下游磷酸化细胞外信号调节激酶 (ERK) (pERK) 水平。虽然 β 界面 R41E/K42D 双突变不会干扰二聚化,但它们几乎完全阻断 K-Ras4B 介导的 ERK 磷酸化。两种双突变均增加了细胞中下游磷酸化 Akt (pAkt) 水平。 pERK 和 pAkt 水平的变化改变了 ERK 和 Akt 调节的基因表达,例如 EGR1、JUN 和 BCL2L11。这些结果强调了 α-界面在 K-Ras4B 同二聚化中的作用以及 β-表面在效应子结合中的作用。 MD 模拟强调膜和高变区 (HVR) 分别与 K-Ras4B 突变体的 α 和 β 界面相互作用,抑制同源二聚化并可能抑制效应子结合。 两个界面的突变都会干扰丝裂原激活蛋白激酶 (MAPK) 和磷酸肌醇 3 激酶信号传导,但形式和程度不同。我们得出结论,二聚化不是必需的,但可以增强下游 MAPK 信号传导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号