当前位置:
X-MOL 学术
›
Neurobiol. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
APOE alters glucose flux through central carbon pathways in astrocytes.
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.nbd.2020.104742 Holden C Williams 1 , Brandon C Farmer 2 , Margaret A Piron 2 , Adeline E Walsh 2 , Ronald C Bruntz 3 , Matthew S Gentry 4 , Ramon C Sun 5 , Lance A Johnson 1
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.nbd.2020.104742 Holden C Williams 1 , Brandon C Farmer 2 , Margaret A Piron 2 , Adeline E Walsh 2 , Ronald C Bruntz 3 , Matthew S Gentry 4 , Ramon C Sun 5 , Lance A Johnson 1
Affiliation
|
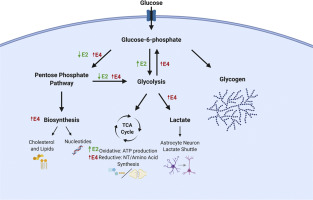
The Apolipoprotein E (APOE) gene is a major genetic risk factor associated with Alzheimer's disease (AD). APOE encodes for three main isoforms in humans (E2, E3, and E4). Homozygous E4 individuals have more than a 10-fold higher risk for developing late-onset AD, while E2 carriers are protected. A hallmark of AD is a reduction in cerebral glucose metabolism, alluding to a strong metabolic component in disease onset and progression. Interestingly, E4 individuals display a similar regional pattern of cerebral glucose hypometabolism decades prior to disease onset. Mapping this metabolic landscape may help elucidate the underlying biological mechanism of APOE-associated risk for AD. Efficient metabolic coupling of neurons and glia is necessary for proper neuronal function, and disruption in glial energy distribution has been proposed to contribute to neuronal cell death and AD pathology. One important function of astrocytes - canonically the primary source of apolipoprotein E in the brain - is to provide metabolic substrates (lactate, lipids, amino acids and neurotransmitters) to neurons. Here we investigate the effects of APOE on astrocyte glucose metabolism in vitro utilizing scintillation proximity assays, stable isotope tracer metabolomics, and gene expression analyses. Glucose uptake is impaired in E4 astrocytes relative to E2 or E3 with specific alterations in central carbon metabolism. Using stable isotope labeled glucose [U-13C] allowed analyses of astrocyte-specific deep metabolic networks affected by APOE, and provided insight to the effects downstream of glucose uptake. Enrichment of 13C in early steps of glycolysis was lowest in E4 astrocytes (highest in E2), while synthesis of lactate from glucose was highest in E4 astrocytes (lowest in E2). We observed an increase in glucose flux through the pentose phosphate pathway (PPP), with downstream increases in gluconeogenesis, lipid, and de novo nucleotide biosynthesis in E4 astrocytes. There was also a marked increase in 13C enrichment in the TCA cycle of E4 astrocytes - whose substrates were also incorporated into biosynthetic pathways at a higher rate. Pyruvate carboxylase (PC) and pyruvate dehydrogenase (PDH) are the two main enzymes controlling pyruvate entry to the TCA cycle. PC gene expression is increased in E4 astrocytes and the activity relative to PDH was also increased, compared to E2 or E3. Decreased enrichment in the TCA cycle of E2 and E3 astrocytes is suggestive of increased oxidation and non-glucose derived anaplerosis, which could be fueling mitochondrial ATP production. Conversely, E4 astrocytes appear to increase carbon flux into the TCA cycle to fuel cataplerosis. Together, these data demonstrate clear APOE isoform-specific effects on glucose utilization in astrocytes, including E4-associated increases in lactate synthesis, PPP flux, and de novo biosynthesis pathways.
中文翻译:

APOE 通过星形胶质细胞中的中央碳通路改变葡萄糖通量。
载脂蛋白 E (APOE) 基因是与阿尔茨海默病 (AD) 相关的主要遗传风险因素。APOE 编码人类的三种主要亚型(E2、E3 和 E4)。纯合 E4 个体发生迟发性 AD 的风险高出 10 倍以上,而 E2 携带者则受到保护。AD 的一个标志是脑葡萄糖代谢减少,暗示疾病发作和进展中的强代谢成分。有趣的是,E4 个体在疾病发作前几十年表现出类似的脑葡萄糖低代谢区域模式。绘制这种代谢图谱可能有助于阐明 APOE 相关的 AD 风险的潜在生物学机制。神经元和神经胶质细胞的有效代谢耦合对于正常的神经元功能是必要的,并且已经提出神经胶质能量分布的破坏有助于神经元细胞死亡和AD病理学。星形胶质细胞(通常是大脑中载脂蛋白 E 的主要来源)的一项重要功能是为神经元提供代谢底物(乳酸、脂质、氨基酸和神经递质)。在这里,我们利用闪烁邻近分析、稳定同位素示踪代谢组学和基因表达分析研究了 APOE 对体外星形胶质细胞葡萄糖代谢的影响。相对于 E2 或 E3,E4 星形胶质细胞的葡萄糖摄取受损,中央碳代谢发生特定变化。使用稳定同位素标记的葡萄糖 [U-13C] 可以分析受 APOE 影响的星形胶质细胞特异性深层代谢网络,并深入了解葡萄糖摄取下游的影响。在 E4 星形胶质细胞中,糖酵解早期步骤中 13C 的富集最低(在 E2 中最高),而在 E4 星形胶质细胞中从葡萄糖合成乳酸最高(在 E2 中最低)。我们观察到通过戊糖磷酸途径 (PPP) 的葡萄糖通量增加,下游 E4 星形胶质细胞中的糖异生、脂质和从头核苷酸生物合成增加。E4星形胶质细胞的TCA循环中的13C富集也显着增加——其底物也以更高的速率掺入生物合成途径中。丙酮酸羧化酶 (PC) 和丙酮酸脱氢酶 (PDH) 是控制丙酮酸进入 TCA 循环的两种主要酶。与 E2 或 E3 相比,E4 星形胶质细胞中 PC 基因表达增加,相对于 PDH 的活性也增加。E2 和 E3 星形胶质细胞 TCA 循环的富集减少表明氧化增加和非葡萄糖衍生的回补,这可能会促进线粒体 ATP 的产生。相反,E4 星形胶质细胞似乎增加了进入 TCA 循环的碳通量,从而促进了代谢。总之,这些数据证明了对星形胶质细胞中葡萄糖利用的明显 APOE 异构体特异性影响,包括与 E4 相关的乳酸合成增加、PPP 通量和从头生物合成途径。
更新日期:2020-01-11
中文翻译:

APOE 通过星形胶质细胞中的中央碳通路改变葡萄糖通量。
载脂蛋白 E (APOE) 基因是与阿尔茨海默病 (AD) 相关的主要遗传风险因素。APOE 编码人类的三种主要亚型(E2、E3 和 E4)。纯合 E4 个体发生迟发性 AD 的风险高出 10 倍以上,而 E2 携带者则受到保护。AD 的一个标志是脑葡萄糖代谢减少,暗示疾病发作和进展中的强代谢成分。有趣的是,E4 个体在疾病发作前几十年表现出类似的脑葡萄糖低代谢区域模式。绘制这种代谢图谱可能有助于阐明 APOE 相关的 AD 风险的潜在生物学机制。神经元和神经胶质细胞的有效代谢耦合对于正常的神经元功能是必要的,并且已经提出神经胶质能量分布的破坏有助于神经元细胞死亡和AD病理学。星形胶质细胞(通常是大脑中载脂蛋白 E 的主要来源)的一项重要功能是为神经元提供代谢底物(乳酸、脂质、氨基酸和神经递质)。在这里,我们利用闪烁邻近分析、稳定同位素示踪代谢组学和基因表达分析研究了 APOE 对体外星形胶质细胞葡萄糖代谢的影响。相对于 E2 或 E3,E4 星形胶质细胞的葡萄糖摄取受损,中央碳代谢发生特定变化。使用稳定同位素标记的葡萄糖 [U-13C] 可以分析受 APOE 影响的星形胶质细胞特异性深层代谢网络,并深入了解葡萄糖摄取下游的影响。在 E4 星形胶质细胞中,糖酵解早期步骤中 13C 的富集最低(在 E2 中最高),而在 E4 星形胶质细胞中从葡萄糖合成乳酸最高(在 E2 中最低)。我们观察到通过戊糖磷酸途径 (PPP) 的葡萄糖通量增加,下游 E4 星形胶质细胞中的糖异生、脂质和从头核苷酸生物合成增加。E4星形胶质细胞的TCA循环中的13C富集也显着增加——其底物也以更高的速率掺入生物合成途径中。丙酮酸羧化酶 (PC) 和丙酮酸脱氢酶 (PDH) 是控制丙酮酸进入 TCA 循环的两种主要酶。与 E2 或 E3 相比,E4 星形胶质细胞中 PC 基因表达增加,相对于 PDH 的活性也增加。E2 和 E3 星形胶质细胞 TCA 循环的富集减少表明氧化增加和非葡萄糖衍生的回补,这可能会促进线粒体 ATP 的产生。相反,E4 星形胶质细胞似乎增加了进入 TCA 循环的碳通量,从而促进了代谢。总之,这些数据证明了对星形胶质细胞中葡萄糖利用的明显 APOE 异构体特异性影响,包括与 E4 相关的乳酸合成增加、PPP 通量和从头生物合成途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号