当前位置:
X-MOL 学术
›
Neurobiol. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondrial fission in Huntington's disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca2+ efflux and Reactive Oxygen Species (ROS) homeostasis.
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.nbd.2020.104741 Marta Cherubini 1 , Laura Lopez-Molina 1 , Silvia Gines 1
Neurobiology of Disease ( IF 5.1 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.nbd.2020.104741 Marta Cherubini 1 , Laura Lopez-Molina 1 , Silvia Gines 1
Affiliation
|
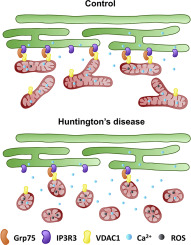
Mitochondria-associated membranes (MAMs) are dynamic structures that communicate endoplasmic reticulum (ER) and mitochondria allowing calcium transfer between these two organelles. Since calcium dysregulation is an important hallmark of several neurodegenerative diseases, disruption of MAMs has been speculated to contribute to pathological features associated with these neurodegenerative processes. In Huntington's disease (HD), mutant huntingtin induces the selective loss of medium spiny neurons within the striatum. The cause of this specific susceptibility remain unclear. However, defects on mitochondrial dynamics and bioenergetics have been proposed as critical contributors, causing accumulation of fragmented mitochondria and subsequent Ca2+ homeostasis alterations. In the present work, we show that aberrant Drp1-mediated mitochondrial fragmentation within the striatum of HD mutant mice, forces mitochondria to place far away from the ER disrupting the ER-mitochondria association and therefore causing drawbacks in Ca2+ efflux and an excessive production of mitochondria superoxide species. Accordingly, inhibition of Drp1 activity by Mdivi-1 treatment restored ER-mitochondria contacts, mitochondria dysfunction and Ca2+ homeostasis. In sum, our results give new insight on how defects on mitochondria dynamics may contribute to striatal vulnerability in HD and highlights MAMs dysfunction as an important factor involved in HD striatal pathology.
中文翻译:

亨廷顿氏病小鼠纹状体中的线粒体裂变破坏了ER线粒体接触,导致Ca2 +外排和活性氧(ROS)稳态失调。
线粒体相关膜(MAMs)是动态结构,可传递内质网(ER)和线粒体,从而允许钙在这两个细胞器之间转移。由于钙失调是几种神经退行性疾病的重要标志,因此推测MAM的破坏会导致与这些神经退行性过程相关的病理特征。在亨廷顿舞蹈病(HD)中,亨廷顿突变体诱导纹状体中的中棘神经元选择性丢失。这种特异性敏感性的原因仍不清楚。然而,线粒体动力学和生物能学的缺陷已被认为是关键的因素,导致线粒体碎片的积累和随后的Ca2 +稳态改变。在目前的工作中,我们表明,HD突变小鼠纹状体中异常的Drp1介导的线粒体片段化,迫使线粒体远离ER放置,从而扰乱了ER-线粒体缔合,因此导致Ca2 +外流缺陷和线粒体超氧化物种的过量生产。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAMs功能障碍是HD纹状体病理学的重要因素。迫使线粒体远离ER放置,从而破坏ER线粒体缔合,从而导致Ca2 +外流缺陷和线粒体超氧化物类的过量产生。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAM功能障碍是HD纹状体病理学的重要因素。迫使线粒体远离ER放置,从而破坏ER线粒体缔合,从而导致Ca2 +外流缺陷和线粒体超氧化物类的过量产生。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAM功能障碍是HD纹状体病理学的重要因素。
更新日期:2020-01-11
中文翻译:

亨廷顿氏病小鼠纹状体中的线粒体裂变破坏了ER线粒体接触,导致Ca2 +外排和活性氧(ROS)稳态失调。
线粒体相关膜(MAMs)是动态结构,可传递内质网(ER)和线粒体,从而允许钙在这两个细胞器之间转移。由于钙失调是几种神经退行性疾病的重要标志,因此推测MAM的破坏会导致与这些神经退行性过程相关的病理特征。在亨廷顿舞蹈病(HD)中,亨廷顿突变体诱导纹状体中的中棘神经元选择性丢失。这种特异性敏感性的原因仍不清楚。然而,线粒体动力学和生物能学的缺陷已被认为是关键的因素,导致线粒体碎片的积累和随后的Ca2 +稳态改变。在目前的工作中,我们表明,HD突变小鼠纹状体中异常的Drp1介导的线粒体片段化,迫使线粒体远离ER放置,从而扰乱了ER-线粒体缔合,因此导致Ca2 +外流缺陷和线粒体超氧化物种的过量生产。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAMs功能障碍是HD纹状体病理学的重要因素。迫使线粒体远离ER放置,从而破坏ER线粒体缔合,从而导致Ca2 +外流缺陷和线粒体超氧化物类的过量产生。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAM功能障碍是HD纹状体病理学的重要因素。迫使线粒体远离ER放置,从而破坏ER线粒体缔合,从而导致Ca2 +外流缺陷和线粒体超氧化物类的过量产生。因此,通过Mdivi-1处理抑制Drp1活性可恢复ER-线粒体接触,线粒体功能障碍和Ca2 +稳态。总之,我们的结果提供了关于线粒体动力学缺陷如何导致HD纹状体易损性的新见解,并强调了MAM功能障碍是HD纹状体病理学的重要因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号