当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supramolecular organisation of sulphate salt hydrates exemplified with brucine sulphate
CrystEngComm ( IF 2.6 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9ce01762c Doris E. Braun 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9ce01762c Doris E. Braun 1, 2, 3, 4
Affiliation
|
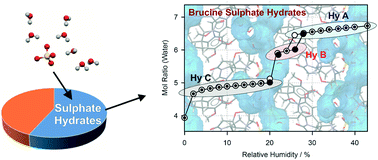
The solid form landscape of brucine sulphate (BS) was elucidated, resulting in three hydrate forms (HyA–C) and amorphous BS. Interconversion of the hydrates of BS with small changes in the relative humidity complicated identifying and characterising the solid forms. The hydrate obtained from crystallisation experiments (from water), HyA, is the only solid form described in the literature. The other two hydrates were produced by dehydration starting from the known hydrate. HyA contains 6.5 to 7.4 molecules of water per BS and is only stable in the relative humidity (RH) range ≥26% at room temperature (RT). HyB is only observable in a very narrow RH window (22–25%) at RT and shows a hexahydrate stoichiometry. At RH values ≤20%, the third hydrate, HyC, forms. Similar to HyA, the latter hydrate shows a variable water content of five or less water molecules per BS. Removal of the essential water molecules stabilising the hydrate structures causes the collapse to the amorphous state, a process which was not completed within 3.5 years of storing HyC under driest conditions (approx. 0%) at room temperature. Only the combination of intermolecular interaction and electronic structure calculations with thermal analytical techniques, X-ray diffraction, IR spectroscopy and gravimetric moisture (de)sorption studies and careful control of the external conditions allowed the discovery and rationalisation of the three hydrates of BS. The investigations on BS were complemented with an exploitation of the Cambridge Structural Database (CSD) to unravel the incidence of hydrates of sulphate salts. The analysis indicates that 56.5% of the sulphate salts (C, H, N, O, and S atoms only) are hydrate structures, with higher hydrates being more commonly present amongst sulphate salts than amongst all organic hydrates.
中文翻译:

硫酸盐水合物的超分子组织形式
马钱子碱硫酸盐(所述的固体形式景观BS)中的溶液阐明,导致3种水合物形式(HYA - C ^)和无定形BS。BS水合物的相互转化以及相对湿度的微小变化使固体形式的鉴定和表征变得复杂。从结晶实验(从水中)得到的水合物HyA是文献中描述的唯一固体形式。其他两种水合物是从已知的水合物开始通过脱水制得的。HyA每BS包含6.5至7.4分子水,并且仅在室温(RT)的相对湿度(RH)范围≥26%时稳定。乙型肝炎只能在室温下在非常窄的相对湿度窗口(22–25%)中观察到,并且显示六水合物化学计量。在RH值≤20%时,形成第三种水合物HyC。与HyA相似,后者的水合物在每个BS中的水含量变化不超过5个或更少。去除稳定水合物结构的基本水分子会导致塌陷至无定形状态,该过程在储存HyC的3.5年内未完成在室温下最干燥的条件下(约0%)。只有分子间相互作用和电子结构计算与热分析技术,X射线衍射,IR光谱和重量水分(脱)吸附研究以及对外部条件的仔细控制相结合,才能发现和合理化BS的三种水合物。对BS的研究得到了剑桥结构数据库(CSD)的补充,以揭示硫酸盐水合物的发生率。分析表明,有56.5%的硫酸盐(仅C,H,N,O和S原子)是水合物结构,与所有有机水合物相比,硫酸盐中更常见的是更高的水合物。
更新日期:2020-02-13
中文翻译:

硫酸盐水合物的超分子组织形式
马钱子碱硫酸盐(所述的固体形式景观BS)中的溶液阐明,导致3种水合物形式(HYA - C ^)和无定形BS。BS水合物的相互转化以及相对湿度的微小变化使固体形式的鉴定和表征变得复杂。从结晶实验(从水中)得到的水合物HyA是文献中描述的唯一固体形式。其他两种水合物是从已知的水合物开始通过脱水制得的。HyA每BS包含6.5至7.4分子水,并且仅在室温(RT)的相对湿度(RH)范围≥26%时稳定。乙型肝炎只能在室温下在非常窄的相对湿度窗口(22–25%)中观察到,并且显示六水合物化学计量。在RH值≤20%时,形成第三种水合物HyC。与HyA相似,后者的水合物在每个BS中的水含量变化不超过5个或更少。去除稳定水合物结构的基本水分子会导致塌陷至无定形状态,该过程在储存HyC的3.5年内未完成在室温下最干燥的条件下(约0%)。只有分子间相互作用和电子结构计算与热分析技术,X射线衍射,IR光谱和重量水分(脱)吸附研究以及对外部条件的仔细控制相结合,才能发现和合理化BS的三种水合物。对BS的研究得到了剑桥结构数据库(CSD)的补充,以揭示硫酸盐水合物的发生率。分析表明,有56.5%的硫酸盐(仅C,H,N,O和S原子)是水合物结构,与所有有机水合物相比,硫酸盐中更常见的是更高的水合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号