当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational and Reaction Dynamic Coupling in Histidine Kinases: Insights from Hybrid QM/MM Simulations.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.jcim.9b00806 Federico A Olivieri 1, 2 , Osvaldo Burastero 1, 2 , Salvador I Drusin 1, 2, 3 , Lucas A Defelipe 1, 2, 4 , Diana E Wetzler 1, 2 , Adrián Turjanski 1, 2 , Marcelo Marti 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.jcim.9b00806 Federico A Olivieri 1, 2 , Osvaldo Burastero 1, 2 , Salvador I Drusin 1, 2, 3 , Lucas A Defelipe 1, 2, 4 , Diana E Wetzler 1, 2 , Adrián Turjanski 1, 2 , Marcelo Marti 1, 2
Affiliation
|
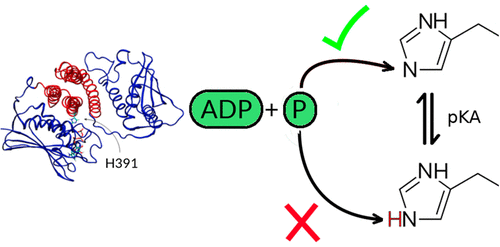
Histidine kinases (HK) of bacterial two-component systems represent a hallmark of allosterism in proteins, being able to detect a signal through the sensor domain and transmit this information through the protein matrix to the kinase domain which, once active, autophosphorylates a specific histidine residue. Inactive-to-active transition results in a large conformational change that moves the kinase on top of the histidine. In the present work, we use several molecular simulation techniques (Molecular Dynamics, Hybrid QM/MM, and constant pH molecular dynamics) to study the activation and autophosphorylation reactions in L. plantarum WalK, a cis-acting HK. In agreement with previous results, we show that the chemical step requires tight coupling with the conformational step in order to maintain the histidine phosphoacceptor in the correct tautomeric state, with a reactive δ-nitrogen. During the conformational transition, the kinase domain is never released and walks along the HK helix axis, breaking and forming several conserved residue-based contacts. The phosphate transfer reaction is concerted in the transition state region and is catalyzed through the stabilization of the negative developing charge of transferring phosphate along the reaction.
中文翻译:

组氨酸激酶的构象和反应动力学偶联:来自混合QM / MM模拟的见解。
细菌两组分系统的组氨酸激酶(HK)代表蛋白质中的变构特征,能够通过传感器结构域检测信号,并将此信息通过蛋白质基质传递至激酶结构域,一旦激活,该结构域就会自磷酸化特定的组氨酸残留物。无活性到活性的过渡导致大的构象变化,使激酶移动到组氨酸的顶部。在当前的工作中,我们使用几种分子模拟技术(分子动力学,混合QM / MM和恒定pH分子动力学)来研究植物乳杆菌WalK(一种顺式作用的HK)的活化和自磷酸化反应。与先前的结果一致,我们表明,化学步骤需要与构象步骤紧密偶联,以使组氨酸磷酸受体保持在正确的互变异构状态,并具有反应性δ-氮。在构象过渡过程中,激酶结构域从未释放,并沿着HK螺旋轴行走,破坏并形成了几个保守的基于残基的接触。磷酸盐转移反应在过渡态区域协调进行,并通过稳定沿反应转移磷酸盐的负显影电荷来催化。
更新日期:2020-02-06
中文翻译:

组氨酸激酶的构象和反应动力学偶联:来自混合QM / MM模拟的见解。
细菌两组分系统的组氨酸激酶(HK)代表蛋白质中的变构特征,能够通过传感器结构域检测信号,并将此信息通过蛋白质基质传递至激酶结构域,一旦激活,该结构域就会自磷酸化特定的组氨酸残留物。无活性到活性的过渡导致大的构象变化,使激酶移动到组氨酸的顶部。在当前的工作中,我们使用几种分子模拟技术(分子动力学,混合QM / MM和恒定pH分子动力学)来研究植物乳杆菌WalK(一种顺式作用的HK)的活化和自磷酸化反应。与先前的结果一致,我们表明,化学步骤需要与构象步骤紧密偶联,以使组氨酸磷酸受体保持在正确的互变异构状态,并具有反应性δ-氮。在构象过渡过程中,激酶结构域从未释放,并沿着HK螺旋轴行走,破坏并形成了几个保守的基于残基的接触。磷酸盐转移反应在过渡态区域协调进行,并通过稳定沿反应转移磷酸盐的负显影电荷来催化。









































 京公网安备 11010802027423号
京公网安备 11010802027423号