当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Structural Contributions to Thermal Adaptation of the TATA-Box Binding Protein: Molecular Dynamics and QSPR Analyses.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jcim.9b00824 Ángel Santiago 1 , Rodrigo Said Razo-Hernández 1 , Nina Pastor 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jcim.9b00824 Ángel Santiago 1 , Rodrigo Said Razo-Hernández 1 , Nina Pastor 1, 2
Affiliation
|
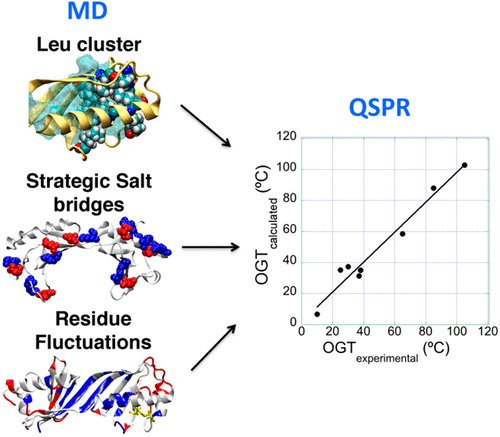
The TATA-box binding protein (TBP) is an important element of the transcription machinery in archaea and eukaryotic organisms. TBP is expressed in organisms adapted to different temperatures, indicating a robust structure, and experimental studies have shown that the mid-unfolding temperature (Tm) of TBP is directly correlated with the optimal growth temperature (OGT) of the organism. To understand which are the relevant structural requirements for its stability, we present the first structural and dynamic computational study of TBPs, combining molecular dynamics (MD) simulations and a quantitative structure-property relationship (QSPR) over a set of TBPs of organisms adapted to different temperatures. We found that the main structural properties of TBP used to adapt to high temperatures are an increase in the ease of desolvation of charged residues at the surface, an increase in the local resiliency, the presence of Leu clusters in the protein core, and an increase in the loss of hydrophobic packing in the N-terminal subdomain. In view of our results, we consider that TBP is a good model to study thermal adaptation, and our analysis opens the possibility of performing protein engineering on TBPs to study transcription at high or low temperatures.
中文翻译:

揭示TATA-Box结合蛋白对热适应的结构贡献:分子动力学和QSPR分析。
TATA-box结合蛋白(TBP)是古细菌和真核生物中转录机制的重要组成部分。TBP在适应不同温度的生物中表达,表明其结构坚固,实验研究表明,TBP的中间展开温度(Tm)与该生物的最佳生长温度(OGT)直接相关。为了了解哪些是对其稳定性的相关结构要求,我们介绍了TBP的第一个结构和动态计算研究,将分子动力学(MD)模拟和定量结构-属性关系(QSPR)结合到一组适应于生物的TBP上不同的温度。我们发现用于适应高温的TBP的主要结构性质是表面带电残基的去溶剂化容易程度增加,局部弹性增加,蛋白核中Leu簇的存在以及增加N-末端亚结构域中疏水性堆积的损失。根据我们的结果,我们认为TBP是研究热适应性的好模型,我们的分析为在TBP上进行蛋白质工程研究高温或低温下的转录开辟了可能性。
更新日期:2020-01-23
中文翻译:

揭示TATA-Box结合蛋白对热适应的结构贡献:分子动力学和QSPR分析。
TATA-box结合蛋白(TBP)是古细菌和真核生物中转录机制的重要组成部分。TBP在适应不同温度的生物中表达,表明其结构坚固,实验研究表明,TBP的中间展开温度(Tm)与该生物的最佳生长温度(OGT)直接相关。为了了解哪些是对其稳定性的相关结构要求,我们介绍了TBP的第一个结构和动态计算研究,将分子动力学(MD)模拟和定量结构-属性关系(QSPR)结合到一组适应于生物的TBP上不同的温度。我们发现用于适应高温的TBP的主要结构性质是表面带电残基的去溶剂化容易程度增加,局部弹性增加,蛋白核中Leu簇的存在以及增加N-末端亚结构域中疏水性堆积的损失。根据我们的结果,我们认为TBP是研究热适应性的好模型,我们的分析为在TBP上进行蛋白质工程研究高温或低温下的转录开辟了可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号