当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion Effect on Gas Absorption in Imidazolium-Based Ionic Liquids.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jcim.9b00885 Jessé G Neumann 1 , Hubert Stassen 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jcim.9b00885 Jessé G Neumann 1 , Hubert Stassen 1
Affiliation
|
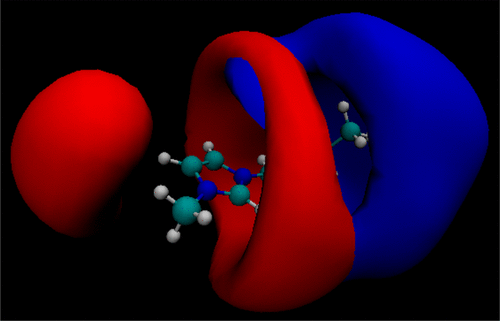
We performed classical Molecular Dynamics computer simulations to analyze solutions of the gases CO2, N2, and CH4 in four 1-n-butyl-3-methylimidazolium-based ionic liquids (1-n-butyl-3-methylimidazolium acetate, 1-n-butyl-3-methylimidazolium prolinate, 1-n-butyl-3-methylimidazolium bromide, and 1-n-butyl-3-methylimidazolium tetrafluoroborate). Typical experimental conditions (10 bar gas pressure and room temperature) have been chosen to study mixtures of the ionic liquids with the gases at a single gas molar fraction of 0.25. Structural aspects are discussed to judge the absorption capacities of the ionic liquids. We observed that CO2 coordinates preferentially within the polar domain of the ionic liquids with the bromide and tetrafluoroborate anions presenting the best performances. The other gases, N2 and CH4, remain in the less polar domains of the ionic liquids. Cluster size analysis indicates phase separation for these two gases. Considering both, the absorption tendency and gas separation capacity of the ionic liquids, the anion is desired to be small and possessing multiple coordination sites. In this aspect, the tetrafluoroborate anion accomplished the best results.
中文翻译:

阴离子对咪唑鎓离子液体中气体吸收的影响。
我们进行了经典的分子动力学计算机模拟,以分析四种基于1-n-丁基-3-甲基咪唑鎓的离子液体(1-n-丁基-3-甲基咪唑乙酸酯,1-n-脯氨酸-3-甲基咪唑鎓丁基酯,溴化1-正丁基-3-甲基咪唑鎓和四氟硼酸1-正丁基-3-甲基咪唑鎓。已选择典型的实验条件(10 bar气压和室温)来研究离子液体与气体在0.25的单个气体摩尔分数下的混合物。讨论了结构方面以判断离子液体的吸收能力。我们观察到,CO2优先在离子液体的极性域内配位,其中溴离子和四氟硼酸根阴离子表现出最佳性能。其他气体N2和CH4 保留在离子液体的极性较小的区域中。团簇尺寸分析表明这两种气体的相分离。考虑到离子液体的吸收趋势和气体分离能力,期望阴离子小并且具有多个配位点。在这方面,四氟硼酸根阴离子实现了最佳结果。
更新日期:2020-01-17
中文翻译:

阴离子对咪唑鎓离子液体中气体吸收的影响。
我们进行了经典的分子动力学计算机模拟,以分析四种基于1-n-丁基-3-甲基咪唑鎓的离子液体(1-n-丁基-3-甲基咪唑乙酸酯,1-n-脯氨酸-3-甲基咪唑鎓丁基酯,溴化1-正丁基-3-甲基咪唑鎓和四氟硼酸1-正丁基-3-甲基咪唑鎓。已选择典型的实验条件(10 bar气压和室温)来研究离子液体与气体在0.25的单个气体摩尔分数下的混合物。讨论了结构方面以判断离子液体的吸收能力。我们观察到,CO2优先在离子液体的极性域内配位,其中溴离子和四氟硼酸根阴离子表现出最佳性能。其他气体N2和CH4 保留在离子液体的极性较小的区域中。团簇尺寸分析表明这两种气体的相分离。考虑到离子液体的吸收趋势和气体分离能力,期望阴离子小并且具有多个配位点。在这方面,四氟硼酸根阴离子实现了最佳结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号