当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Transfer in Rhodamine–TiO2 Complexes Studied as a Function of Chalcogen and Bridge Substitution
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b11049 Zachary Piontkowski 1 , Yu-Chen Wang 2 , Yu-Xiu Liu 2 , Yi Zhao 2 , David W. McCamant 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b11049 Zachary Piontkowski 1 , Yu-Chen Wang 2 , Yu-Xiu Liu 2 , Yi Zhao 2 , David W. McCamant 1
Affiliation
|
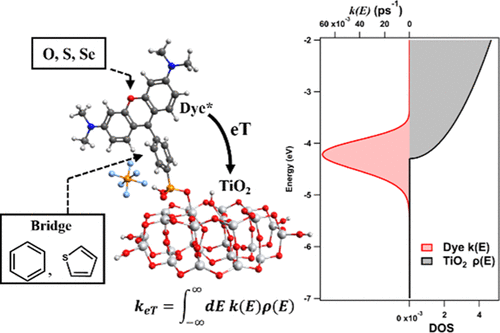
Many emerging light-harvesting systems for solar-energy capture depend on absorption of light by molecular dyes and subsequent electron transfer to metal-oxide semiconductors. However, the inhomogeneous electron-transfer process is often misunderstood when analogies from bimolecular electron transfer are used to explain experimental trends. Here, we develop and apply a theoretical methodology that correctly incorporates the semiconductor density of states and the system reorganization energies to explain observed trends in a series of molecular sensitizers. The effects of chalcogen and bridge substitution on the electron transfer in rhodamine–TiO2 complexes are theoretically investigated by combining density functional theory (DFT)/time-dependent DFT calculations and Fermi’s golden rule for the rate constant. It is shown that all dyes exhibit τeT < 4 ps. Dyes with thiophene bridges exhibit shorter τeT (∼1 ps) than dyes with phenylene bridges (∼4 ps). When the planes of the dye core and bridge are fixed at coplanarity, the dye–TiO2 coupling strength is found to increase by a factor of ∼2 when compared with the Franck–Condon geometry. However, the donor energy level of coplanar dyes falls significantly below the TiO2 conduction band edge so that, despite enhanced coupling, electron transfer is slowed to ∼20 ps. Similar results appear for the excited triplet states of these dyes, showing that the intersystem crossing to low energy triplet states can increase electron-transfer time constants to 60–240 ps. These results are compared to the results of previous photocatalytic hydrogen generation and dye-sensitized solar cell experiments.
中文翻译:

罗丹明-TiO 2配合物中电子转移与硫族元素和桥取代作用的关系
许多新兴的用于太阳能捕获的光收集系统取决于分子染料对光的吸收以及随后电子向金属氧化物半导体的转移。但是,当使用双分子电子转移的类比来解释实验趋势时,常常会误解不均匀的电子转移过程。在这里,我们开发并应用了一种理论方法,该方法正确地结合了半导体的态密度和系统重组能来解释一系列分子敏化剂中观察到的趋势。硫族元素和桥取代对若丹明-TiO 2中电子转移的影响通过结合密度泛函理论(DFT)/时间相关DFT计算和费米黄金定律的速率常数,对复合物进行了理论研究。结果表明,所有染料的τeT <4 ps。带有噻吩桥的染料比带有亚苯基桥的染料(〜4 ps)具有更短的τeT(〜1 ps)。当染料核和桥的平面共面固定时,与Franck-Condon几何相比,染料-TiO 2的耦合强度增加了约2倍。但是,共面染料的供体能级明显低于TiO 2导带边缘,因此,尽管增强了耦合,但电子传输仍会减慢至约20 ps。这些染料的激发三重态表现出相似的结果,表明与低能三重态交叉的系统间可以将电子转移时间常数增加到60-240 ps。将这些结果与以前的光催化制氢和染料敏化太阳能电池实验的结果进行了比较。
更新日期:2020-01-23
中文翻译:

罗丹明-TiO 2配合物中电子转移与硫族元素和桥取代作用的关系
许多新兴的用于太阳能捕获的光收集系统取决于分子染料对光的吸收以及随后电子向金属氧化物半导体的转移。但是,当使用双分子电子转移的类比来解释实验趋势时,常常会误解不均匀的电子转移过程。在这里,我们开发并应用了一种理论方法,该方法正确地结合了半导体的态密度和系统重组能来解释一系列分子敏化剂中观察到的趋势。硫族元素和桥取代对若丹明-TiO 2中电子转移的影响通过结合密度泛函理论(DFT)/时间相关DFT计算和费米黄金定律的速率常数,对复合物进行了理论研究。结果表明,所有染料的τeT <4 ps。带有噻吩桥的染料比带有亚苯基桥的染料(〜4 ps)具有更短的τeT(〜1 ps)。当染料核和桥的平面共面固定时,与Franck-Condon几何相比,染料-TiO 2的耦合强度增加了约2倍。但是,共面染料的供体能级明显低于TiO 2导带边缘,因此,尽管增强了耦合,但电子传输仍会减慢至约20 ps。这些染料的激发三重态表现出相似的结果,表明与低能三重态交叉的系统间可以将电子转移时间常数增加到60-240 ps。将这些结果与以前的光催化制氢和染料敏化太阳能电池实验的结果进行了比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号