当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observation of Extremely Weakly Interacting OH (∼3600 cm–1) in the Vicinity of High Charge Density Metal Ions (Mz+; z = 1, 2, 3): A Structural Heterogeneity in the Extended Hydration Shell
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b09692 Animesh Patra 1 , Subhadip Roy 2 , Subhamoy Saha 2 , Dipak K. Palit 1 , Jahur A. Mondal 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jpcc.9b09692 Animesh Patra 1 , Subhadip Roy 2 , Subhamoy Saha 2 , Dipak K. Palit 1 , Jahur A. Mondal 2
Affiliation
|
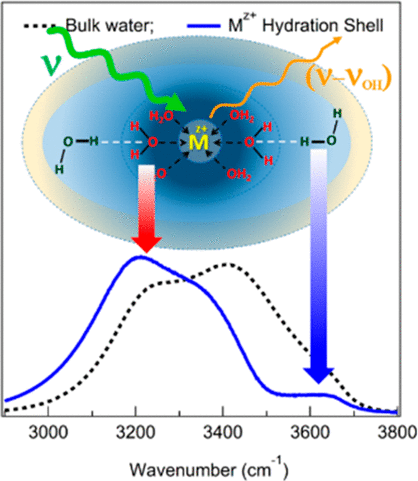
Hydration of metal ions is critical to their adsorption, complexation, and discharge but remained elusive due to counterion interference. We introduce Raman difference spectroscopy with simultaneous curve fitting (RD-SCF) analysis to quantitatively retrieve the counterion-free OH stretch spectra of water in the hydration shell of various metal ions such as Li+, Mg2+, Ca2+, Sr2+, Ba2+, La3+, Gd3+ Dy3+, and Lu3+ as well as that of a proton (H+). The RD-SCF-extracted spectra show that “extremely weakly” (OH stretch ∼ 3600 cm–1) and “strongly” (∼3300 cm–1) interacting OHs coexist in the hydration shell of the high charge density metal ions and proton. The weakly interacting OH originates from the second hydration shell water–OH (W2hs–OH) that donates the hydrogen-bond to the electron-deficient oxygen at the first hydration shell (W1hs−O) and is largely localized at the boundary between the first and second hydration shells. For high charge density ions, such as Mg2+ and La3+, at least 4 and 8 water molecules are affected in their respective second hydration shell, which is ∼70% and 90% of their first hydration shell. The coexistence of different water–OHs discloses a heterogeneous water structure in the extended hydration shell of metal ions.
中文翻译:

在高电荷密度金属离子(M z +;z = 1,2,3 )附近观察到极弱相互作用的OH(〜3600 cm –1):扩展水化壳中的结构异质性
金属离子的水合对其吸附,络合和放电至关重要,但由于抗衡离子的干扰而难以捉摸。我们引入具有同时曲线拟合(RD-SCF)分析的拉曼差光谱法,定量检索各种金属离子(如Li +,Mg 2 +,Ca 2 +,Sr 2)的水合壳中水的无反离子OH拉伸谱+,Ba 2 +,La 3+,Gd 3+ Dy 3+和Lu 3+以及质子(H +)的离子。RD-SCF提取的光谱表明,“极弱”(OH拉伸〜3600 cm –1)和“强”(〜3300 cm)–1)相互作用的OHs共存于高电荷密度金属离子和质子的水合壳中。弱相互作用的OH源自第二个水合壳水-OH(W 2hs -OH),它在第一个水合壳(W 1hs -O)上将氢键提供给缺电子的氧,并且主要位于第一和第二水化壳。对于高电荷密度的离子,例如Mg 2+和La 3+,它们各自的第二水合壳中至少有4和8个水分子受到影响,这分别是其第一水合壳的70%和90%。不同水-OH的共存揭示了金属离子扩展水化壳中的异质水结构。
更新日期:2020-01-24
中文翻译:

在高电荷密度金属离子(M z +;z = 1,2,3 )附近观察到极弱相互作用的OH(〜3600 cm –1):扩展水化壳中的结构异质性
金属离子的水合对其吸附,络合和放电至关重要,但由于抗衡离子的干扰而难以捉摸。我们引入具有同时曲线拟合(RD-SCF)分析的拉曼差光谱法,定量检索各种金属离子(如Li +,Mg 2 +,Ca 2 +,Sr 2)的水合壳中水的无反离子OH拉伸谱+,Ba 2 +,La 3+,Gd 3+ Dy 3+和Lu 3+以及质子(H +)的离子。RD-SCF提取的光谱表明,“极弱”(OH拉伸〜3600 cm –1)和“强”(〜3300 cm)–1)相互作用的OHs共存于高电荷密度金属离子和质子的水合壳中。弱相互作用的OH源自第二个水合壳水-OH(W 2hs -OH),它在第一个水合壳(W 1hs -O)上将氢键提供给缺电子的氧,并且主要位于第一和第二水化壳。对于高电荷密度的离子,例如Mg 2+和La 3+,它们各自的第二水合壳中至少有4和8个水分子受到影响,这分别是其第一水合壳的70%和90%。不同水-OH的共存揭示了金属离子扩展水化壳中的异质水结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号