当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of the Tris(trimethylsilyl)silyl Group on the Fluorescence and Triplet Yields of Oligothiophenes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpcc.9b10718 Shuzo Hirata 1 , Masaki Nishio 2 , Hikaru Uchida 2 , Tsukasa Usuki 2 , Toyotaka Nakae 2 , Mariko Miyachi 2 , Yoshinori Yamanoi 2 , Hiroshi Nishihara 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpcc.9b10718 Shuzo Hirata 1 , Masaki Nishio 2 , Hikaru Uchida 2 , Tsukasa Usuki 2 , Toyotaka Nakae 2 , Mariko Miyachi 2 , Yoshinori Yamanoi 2 , Hiroshi Nishihara 2
Affiliation
|
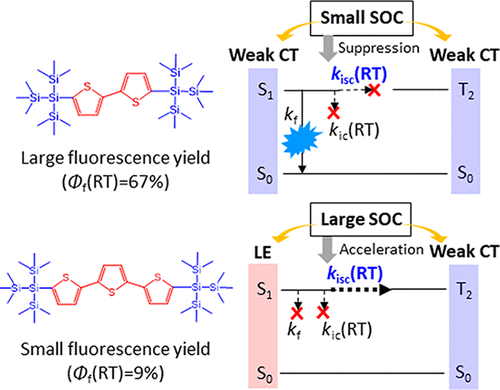
The origin of the large difference of room-temperature fluorescence yields (Φf(RT)) among tris(trimethylsilyl)silylated oligothiophene derivatives was investigated. Tris(trimethylsilyl)silylated thiophene (1) and tris(trimethylsilyl)silylated terthiophene (3) show low fluorescence yields while that of tris(trimethylsilyl)silylated bithiophene (2) is high. Nanosecond transient absorption measurements for 2 and 3 verified that the large difference between their intersystem crossing (ISC) rates from the lowest singlet excited state (S1) causes the large difference in Φf(RT). Quantum calculations indicated that the Si–Si σ bond of (Me3Si)3Si, corresponding to the highest occupied molecular orbital (HOMO), is closely involved in the ISC from S1. The planar conjugated core having much higher or comparable HOMO energy relative to the (Me3Si)3Si substituent, such as 1 and 3, induces large spin–orbit coupling (SOC) between S1 and the second-order triplet excited state (T2), resulting in fast ISC from S1 leading to a small Φf(RT). However, a planar conjugated core having slightly higher HOMO energy than that of the (Me3Si)3Si substituent, such as 2, minimizes SOC between S1 and T2, resulting in slow ISC from S1 leading to a large Φf(RT). Thus, the relationship between the HOMO level of the (Me3Si)3Si substituent and that of the planar conjugated core is key to controlling the ISC from S1.
中文翻译:

三(三甲基甲硅烷基)甲硅烷基对寡聚噻吩的荧光和三重态产率的影响
室温荧光产率的大的差异(Φ的原点˚F三(三甲基硅烷基)甲硅烷基化低聚噻吩衍生物中(RT))进行了研究。三(三甲基甲硅烷基)甲硅烷基噻吩(1)和三(三甲基甲硅烷基)甲硅烷基噻吩(3)显示出低的荧光产率,而三(三甲基甲硅烷基)甲硅烷基化联噻吩(2)则高。纳秒瞬态吸收测量2和3证实了他们的系间窜越(ISC)从最低单重激发态率(S之间的大的差异1)会导致Φ的较大差异˚F(RT)。量子计算表明(Me的Si-Siσ键3 Si)3 Si对应于最高占据分子轨道(HOMO),从S 1紧密参与ISC 。相对于(Me 3 Si)3 Si取代基(例如1和3),HOMO能量高得多或相当的平面共轭核,会引起S 1和二阶三重激发态之间的大自旋轨道耦合(SOC)( Ť 2),导致选自S快速ISC 1导致小Φ ˚F(RT)。但是,平面共轭核的HOMO能量比(Me 3 Si)3 Si取代基的HOMO能量略高2,最小SOC S之间1和T 2,从而产生选自S慢ISC 1导致大Φ ˚F(RT)。因此,(Me 3 Si)3 Si取代基的HOMO能级与平面共轭核的HOMO能级之间的关系是从S 1控制ISC的关键。
更新日期:2020-01-23
中文翻译:

三(三甲基甲硅烷基)甲硅烷基对寡聚噻吩的荧光和三重态产率的影响
室温荧光产率的大的差异(Φ的原点˚F三(三甲基硅烷基)甲硅烷基化低聚噻吩衍生物中(RT))进行了研究。三(三甲基甲硅烷基)甲硅烷基噻吩(1)和三(三甲基甲硅烷基)甲硅烷基噻吩(3)显示出低的荧光产率,而三(三甲基甲硅烷基)甲硅烷基化联噻吩(2)则高。纳秒瞬态吸收测量2和3证实了他们的系间窜越(ISC)从最低单重激发态率(S之间的大的差异1)会导致Φ的较大差异˚F(RT)。量子计算表明(Me的Si-Siσ键3 Si)3 Si对应于最高占据分子轨道(HOMO),从S 1紧密参与ISC 。相对于(Me 3 Si)3 Si取代基(例如1和3),HOMO能量高得多或相当的平面共轭核,会引起S 1和二阶三重激发态之间的大自旋轨道耦合(SOC)( Ť 2),导致选自S快速ISC 1导致小Φ ˚F(RT)。但是,平面共轭核的HOMO能量比(Me 3 Si)3 Si取代基的HOMO能量略高2,最小SOC S之间1和T 2,从而产生选自S慢ISC 1导致大Φ ˚F(RT)。因此,(Me 3 Si)3 Si取代基的HOMO能级与平面共轭核的HOMO能级之间的关系是从S 1控制ISC的关键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号