当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
rac ‐ and meso ‐Cyclohexanoids: Their α ‐, β ‐glycosidases, antibacterial, antifungal activities, and molecular docking studies
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-10 , DOI: 10.1002/ardp.201900267 Emel Karakılıç 1 , Şule Baran 2 , Hatice Öğütçü 3 , Atilla Akdemir 4 , Arif Baran 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-01-10 , DOI: 10.1002/ardp.201900267 Emel Karakılıç 1 , Şule Baran 2 , Hatice Öğütçü 3 , Atilla Akdemir 4 , Arif Baran 1
Affiliation
|
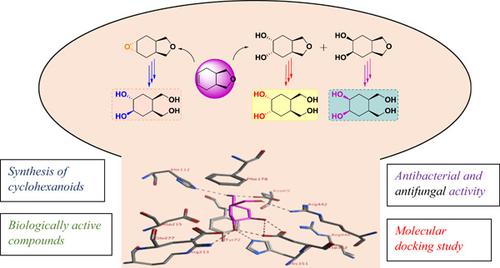
An efficient and versatile synthesis method has been postulated for hydroxymethylated rac‐ and meso‐cyclohexanoid derivatives. The synthesis of these stereoisomers was achieved easily with traditional methods using hexahydroisobenzofuran 6, prepared from commercially available cis‐hydrophthalic anhydride. The study, involving diastereoselective epoxidation and cis‐hydroxylation, was conducted to obtain epoxy‐, cis‐, and trans‐diol‐furans 7, 8, and 9. After sulfamic acid‐catalyzed ring‐opening reaction of the epoxide and furan rings, rac‐ and meso‐tetraacetates 14, 15, and 16 were afforded. Hydrolysis of acetate groups with ammonia in absolute methanol yielded the desired tetrols rac‐17, meso‐18, and meso‐19. All structures, after purification by chromatographic methods and elucidation by spectral techniques, were screened against α‐ and β‐glucosidases. Compounds 7, 8, 10, 17, 18, and 19 were also evaluated for their antibacterial and antifungal activity against some selected synthesized compounds with varying degrees of inhibitory effects on the growth of different pathogenic microorganisms by the well‐diffusion method. In addition, Saccharomyces cerevisiae α‐glucosidase molecular modeling studies were performed for all rac‐ and meso‐compounds 7, 8, 10, 17, 18, and 19.
中文翻译:

外消旋和内消旋环己烷:它们的α-、β-糖苷酶、抗菌、抗真菌活性和分子对接研究
一种有效且通用的合成方法已被假定用于羟甲基化的外消旋和内消旋环己烷衍生物。使用六氢异苯并呋喃 6 的传统方法很容易合成这些立体异构体,六氢异苯并呋喃 6 由市售的顺式氢邻苯二甲酸酐制备。该研究涉及非对映选择性环氧化和顺式羟基化,以获得环氧、顺式和反式二醇-呋喃 7、8 和 9。在环氧化物和呋喃环的氨基磺酸催化开环反应后,得到外消旋和内消旋四乙酸酯 14、15 和 16。在无水甲醇中用氨水解乙酸酯基团产生所需的四醇 rac-17、meso-18 和 meso-19。所有结构,经色谱法纯化和光谱技术阐明后,对α-和β-葡萄糖苷酶进行了筛选。还通过井扩散法评估了化合物 7、8、10、17、18 和 19 对某些选定合成化合物的抗菌和抗真菌活性,这些化合物对不同病原微生物的生长具有不同程度的抑制作用。此外,对所有外消旋和内消旋化合物 7、8、10、17、18 和 19 进行了酿酒酵母 α-葡萄糖苷酶分子建模研究。
更新日期:2020-01-10
中文翻译:

外消旋和内消旋环己烷:它们的α-、β-糖苷酶、抗菌、抗真菌活性和分子对接研究
一种有效且通用的合成方法已被假定用于羟甲基化的外消旋和内消旋环己烷衍生物。使用六氢异苯并呋喃 6 的传统方法很容易合成这些立体异构体,六氢异苯并呋喃 6 由市售的顺式氢邻苯二甲酸酐制备。该研究涉及非对映选择性环氧化和顺式羟基化,以获得环氧、顺式和反式二醇-呋喃 7、8 和 9。在环氧化物和呋喃环的氨基磺酸催化开环反应后,得到外消旋和内消旋四乙酸酯 14、15 和 16。在无水甲醇中用氨水解乙酸酯基团产生所需的四醇 rac-17、meso-18 和 meso-19。所有结构,经色谱法纯化和光谱技术阐明后,对α-和β-葡萄糖苷酶进行了筛选。还通过井扩散法评估了化合物 7、8、10、17、18 和 19 对某些选定合成化合物的抗菌和抗真菌活性,这些化合物对不同病原微生物的生长具有不同程度的抑制作用。此外,对所有外消旋和内消旋化合物 7、8、10、17、18 和 19 进行了酿酒酵母 α-葡萄糖苷酶分子建模研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号