当前位置:
X-MOL 学术
›
npj Vaccines
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heterologous viral protein interactions within licensed seasonal influenza virus vaccines.
npj Vaccines ( IF 9.2 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41541-019-0153-1 Marina Koroleva 1 , Frances Batarse 1 , Savannah Moritzky 1 , Carole Henry 2 , Francisco Chaves 1 , Patrick Wilson 2 , Florian Krammer 3 , Katherine Richards 1 , Andrea J Sant 1
npj Vaccines ( IF 9.2 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41541-019-0153-1 Marina Koroleva 1 , Frances Batarse 1 , Savannah Moritzky 1 , Carole Henry 2 , Francisco Chaves 1 , Patrick Wilson 2 , Florian Krammer 3 , Katherine Richards 1 , Andrea J Sant 1
Affiliation
|
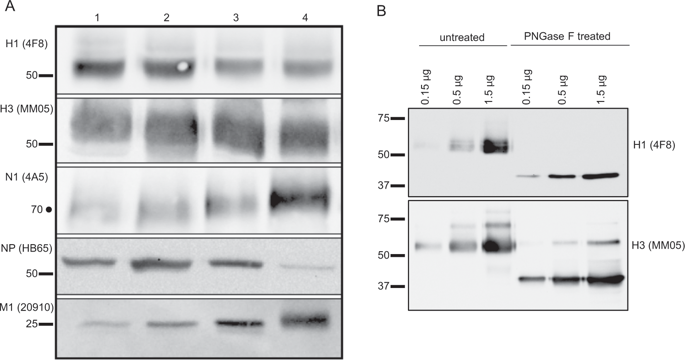
Currently, licensed influenza virus vaccines are designed and tested only for their ability to elicit hemagglutinin (HA)-reactive, neutralizing antibodies. Despite this, the purification process in vaccine manufacturing often does not completely remove other virion components. In the studies reported here, we have examined the viral protein composition of a panel of licensed vaccines from different manufacturers and licensed in different years. Using western blotting, we found that, beyond HA proteins, there are detectable quantities of neuraminidase (NA), nucleoprotein (NP), and matrix proteins (M1) from both influenza A and influenza B viruses in the vaccines but that the composition differed by source and method of vaccine preparation. We also found that disparities in viral protein composition were associated with distinct patterns of elicited antibody specificities. Strikingly, our studies also revealed that many viral proteins contained in the vaccine form heterologous complexes. When H1 proteins were isolated by immunoprecipitation, NA (N1), M1 (M1-A), H3, and HA-B proteins were co-isolated with the H1. Further biochemical studies suggest that these interactions persist for at least 4 h at 37 °C and that the membrane/intracytoplasmic domains in the intact HA proteins are important for the intermolecular interactions detected. These studies indicate that, if such interactions persist after vaccines reach the draining lymph node, both dendritic cells and HA-specific B cells may take up multiple viral proteins simultaneously. Whether these interactions are beneficial or harmful to the developing immune response will depend on the functional potential of the elicited virus-specific CD4 T cells.
中文翻译:

经许可的季节性流感病毒疫苗中的异源病毒蛋白相互作用。
目前,获得许可的流感病毒疫苗仅针对其引发血凝素 (HA) 反应性中和抗体的能力进行设计和测试。尽管如此,疫苗制造中的纯化过程通常不会完全去除其他病毒粒子成分。在此处报告的研究中,我们检查了来自不同制造商并在不同年份获得许可的一组许可疫苗的病毒蛋白组成。使用蛋白质印迹,我们发现,除了 HA 蛋白之外,疫苗中还存在可检测数量的来自甲型流感病毒和乙型流感病毒的神经氨酸酶 (NA)、核蛋白 (NP) 和基质蛋白 (M1),但其成分差异在于疫苗制备的来源和方法。我们还发现病毒蛋白组成的差异与引发的抗体特异性的不同模式有关。引人注目的是,我们的研究还表明,疫苗中包含的许多病毒蛋白形成异源复合物。当通过免疫沉淀分离 H1 蛋白时,NA (N1)、M1 (M1-A)、H3 和 HA-B 蛋白与 H1 共分离。进一步的生化研究表明,这些相互作用在 37°C 下持续至少 4 小时,并且完整 HA 蛋白中的膜/胞质内结构域对于检测到的分子间相互作用很重要。这些研究表明,如果疫苗到达引流淋巴结后这种相互作用持续存在,树突状细胞和 HA 特异性 B 细胞可能同时吸收多种病毒蛋白。
更新日期:2020-01-10
中文翻译:

经许可的季节性流感病毒疫苗中的异源病毒蛋白相互作用。
目前,获得许可的流感病毒疫苗仅针对其引发血凝素 (HA) 反应性中和抗体的能力进行设计和测试。尽管如此,疫苗制造中的纯化过程通常不会完全去除其他病毒粒子成分。在此处报告的研究中,我们检查了来自不同制造商并在不同年份获得许可的一组许可疫苗的病毒蛋白组成。使用蛋白质印迹,我们发现,除了 HA 蛋白之外,疫苗中还存在可检测数量的来自甲型流感病毒和乙型流感病毒的神经氨酸酶 (NA)、核蛋白 (NP) 和基质蛋白 (M1),但其成分差异在于疫苗制备的来源和方法。我们还发现病毒蛋白组成的差异与引发的抗体特异性的不同模式有关。引人注目的是,我们的研究还表明,疫苗中包含的许多病毒蛋白形成异源复合物。当通过免疫沉淀分离 H1 蛋白时,NA (N1)、M1 (M1-A)、H3 和 HA-B 蛋白与 H1 共分离。进一步的生化研究表明,这些相互作用在 37°C 下持续至少 4 小时,并且完整 HA 蛋白中的膜/胞质内结构域对于检测到的分子间相互作用很重要。这些研究表明,如果疫苗到达引流淋巴结后这种相互作用持续存在,树突状细胞和 HA 特异性 B 细胞可能同时吸收多种病毒蛋白。



























 京公网安备 11010802027423号
京公网安备 11010802027423号