当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemodivergent Preparation of Various Heterocycles via Phase‐Transfer Catalysis: Enantioselective Synthesis of Functionalized Piperidines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-30 , DOI: 10.1002/adsc.201901500 Giulio Bertuzzi 1 , Filippo Silvestrini 1 , Pierluigi Moimare 1 , Daniel Pecorari 1 , Andrea Mazzanti 1 , Luca Bernardi 1 , Mariafrancesca Fochi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-30 , DOI: 10.1002/adsc.201901500 Giulio Bertuzzi 1 , Filippo Silvestrini 1 , Pierluigi Moimare 1 , Daniel Pecorari 1 , Andrea Mazzanti 1 , Luca Bernardi 1 , Mariafrancesca Fochi 1
Affiliation
|
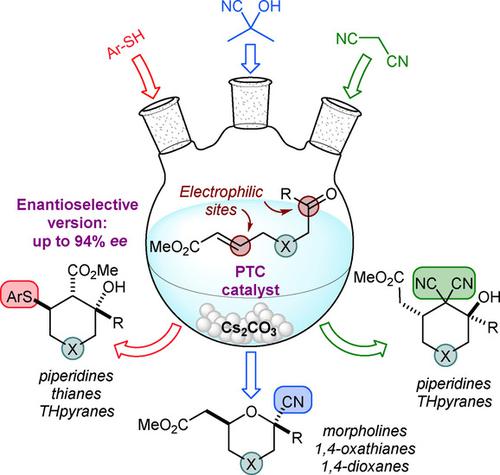
In this work, a new chemodivergent domino approach for the preparation of various saturated heterocycles, based on phase‐transfer catalysis (PTC), is presented. The versatile nature of doubly electrophilic substrates, showing both a Michael acceptor and a ketone, tethered by a heteroatom, enables three different domino reaction pathways. The nucleophile dictates the chemoselectivity of the reaction. Sulfa‐Michael/aldol, cyanide addition/oxa‐Michael and Michael/H‐shift/aldol processes, along with the variation of the tethering heteroatom, results in the formation of six different classes of saturated heterocycles. DFT calculations account for the observed chemo‐ and diastereoselectivity of the two most productive processes. Moreover, an extensive investigation on the sulfa‐Michael/aldol pathway was carried out, ultimately leading to the development of a new enantioselective domino approach to multi‐substituted piperidines based on PTC.
中文翻译:

通过相转移催化化学杂合制备各种杂环化合物:功能化哌啶的对映选择性合成
在这项工作中,提出了一种基于相转移催化(PTC)制备各种饱和杂环的新的化学发散性多米诺骨牌方法。双亲电子底物的多用途性质(同时显示迈克尔受体和酮)被杂原子束缚,实现了三种不同的多米诺反应途径。亲核试剂决定了反应的化学选择性。磺基-迈克尔/羟醛,氰化物加成/氧-迈克尔和迈克尔/ H-移位/羟醛过程,以及束缚杂原子的变化,导致形成六种不同类别的饱和杂环。DFT计算考虑了两个生产率最高的过程中观察到的化学选择性和非对映选择性。此外,对磺胺-迈克尔/醛醇通道进行了广泛的研究,
更新日期:2020-01-31
中文翻译:

通过相转移催化化学杂合制备各种杂环化合物:功能化哌啶的对映选择性合成
在这项工作中,提出了一种基于相转移催化(PTC)制备各种饱和杂环的新的化学发散性多米诺骨牌方法。双亲电子底物的多用途性质(同时显示迈克尔受体和酮)被杂原子束缚,实现了三种不同的多米诺反应途径。亲核试剂决定了反应的化学选择性。磺基-迈克尔/羟醛,氰化物加成/氧-迈克尔和迈克尔/ H-移位/羟醛过程,以及束缚杂原子的变化,导致形成六种不同类别的饱和杂环。DFT计算考虑了两个生产率最高的过程中观察到的化学选择性和非对映选择性。此外,对磺胺-迈克尔/醛醇通道进行了广泛的研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号