当前位置:
X-MOL 学术
›
PLOS Negl. Trop. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structures of Triosephosphate Isomerases from Taenia solium and Schistosoma mansoni provide insights for vaccine rationale and drug design against helminth parasites.
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-01-10 , DOI: 10.1371/journal.pntd.0007815 Pedro Jimenez-Sandoval 1 , Eduardo Castro-Torres 1 , Rogelio González-González 1 , Corina Díaz-Quezada 1 , Misraim Gurrola 1 , Laura D Camacho-Manriquez 1 , Lucia Leyva-Navarro 1 , Luis G Brieba 1
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-01-10 , DOI: 10.1371/journal.pntd.0007815 Pedro Jimenez-Sandoval 1 , Eduardo Castro-Torres 1 , Rogelio González-González 1 , Corina Díaz-Quezada 1 , Misraim Gurrola 1 , Laura D Camacho-Manriquez 1 , Lucia Leyva-Navarro 1 , Luis G Brieba 1
Affiliation
|
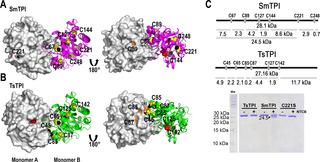
Triosephosphate isomerases (TPIs) from Taenia solium (TsTPI) and Schistosoma mansoni (SmTPI) are potential vaccine and drug targets against cysticercosis and schistosomiasis, respectively. This is due to the dependence of parasitic helminths on glycolysis and because those proteins elicit an immune response, presumably due to their surface localization. Here we report the crystal structures of TsTPI and SmTPI in complex with 2-phosphoglyceric acid (2-PGA). Both TPIs fold into a dimeric (β-α)8 barrel in which the dimer interface consists of α-helices 2, 3, and 4, and swapping of loop 3. TPIs from parasitic helminths harbor a region of three amino acids knows as the SXD/E insert (S155 to E157 and S157 to D159 in TsTPI and SmTPI, respectively). This insert is located between α5 and β6 and is proposed to be the main TPI epitope. This region is part of a solvent-exposed 310-helix that folds into a hook-like structure. The crystal structures of TsTPI and SmTPI predicted conformational epitopes that could be used for vaccine design. Surprisingly, the epitopes corresponding to the SXD/E inserts are not the ones with the greatest immunological potential. SmTPI, but not TsTPI, habors a sole solvent exposed cysteine (SmTPI-S230) and alterations in this residue decrease catalysis. The latter suggests that thiol-conjugating agents could be used to target SmTPI. In sum, the crystal structures of SmTPI and TsTPI are a blueprint for targeted schistosomiasis and cysticercosis drug and vaccine development.
中文翻译:

Ta虫和曼氏血吸虫的磷酸三磷酸异构酶的晶体结构为针对蠕虫寄生虫的疫苗原理和药物设计提供了见识。
来自Ta虫(Tenia solium)(TsTPI)和曼氏血吸虫(SmTPI)的磷酸三糖异构酶(TPIs)分别是针对囊尾rc病和血吸虫病的潜在疫苗和药物靶标。这是由于寄生虫蠕虫对糖酵解的依赖性,以及这些蛋白质引起的免疫反应,大概是由于它们的表面定位所致。在这里我们报告与2-磷酸甘油酸(2-PGA)复杂的TsTPI和SmTPI的晶体结构。两个TPI都折叠成二聚(β-α)8桶,其中二聚体界面由α-螺旋2、3和4组成,并交换环3。来自寄生蠕虫的TPI包含三个氨基酸的区域,称为SXD / E插件(分别在TsTPI和SmTPI中为S155至E157和S157至D159)。该插入片段位于α5和β6之间,被认为是主要的TPI表位。该区域是暴露于溶剂的310螺旋结构的一部分,该螺旋结构折叠成钩状结构。TsTPI和SmTPI的晶体结构预测了可用于疫苗设计的构象表位。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总而言之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。
更新日期:2020-01-10
中文翻译:

Ta虫和曼氏血吸虫的磷酸三磷酸异构酶的晶体结构为针对蠕虫寄生虫的疫苗原理和药物设计提供了见识。
来自Ta虫(Tenia solium)(TsTPI)和曼氏血吸虫(SmTPI)的磷酸三糖异构酶(TPIs)分别是针对囊尾rc病和血吸虫病的潜在疫苗和药物靶标。这是由于寄生虫蠕虫对糖酵解的依赖性,以及这些蛋白质引起的免疫反应,大概是由于它们的表面定位所致。在这里我们报告与2-磷酸甘油酸(2-PGA)复杂的TsTPI和SmTPI的晶体结构。两个TPI都折叠成二聚(β-α)8桶,其中二聚体界面由α-螺旋2、3和4组成,并交换环3。来自寄生蠕虫的TPI包含三个氨基酸的区域,称为SXD / E插件(分别在TsTPI和SmTPI中为S155至E157和S157至D159)。该插入片段位于α5和β6之间,被认为是主要的TPI表位。该区域是暴露于溶剂的310螺旋结构的一部分,该螺旋结构折叠成钩状结构。TsTPI和SmTPI的晶体结构预测了可用于疫苗设计的构象表位。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。令人惊讶地,对应于SXD / E插入片段的表位不是具有最大免疫学潜力的那些。SmTPI,而不是TsTPI,具有暴露于半胱氨酸的唯一溶剂(SmTPI-S230),并且该残基的改变降低了催化作用。后者表明硫醇结合剂可用于靶向SmTPI。总而言之,SmTPI和TsTPI的晶体结构是靶向血吸虫病和囊虫病药物和疫苗开发的蓝图。











































 京公网安备 11010802027423号
京公网安备 11010802027423号