当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structure at the Origin of the Thermal Stability and Large Enthalpy of Fusion and Sublimation Values of Calixarenes
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.cgd.9b01562 Ulises Galindo-García 1 , Luis Alfonso Torres 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-01-17 , DOI: 10.1021/acs.cgd.9b01562 Ulises Galindo-García 1 , Luis Alfonso Torres 1
Affiliation
|
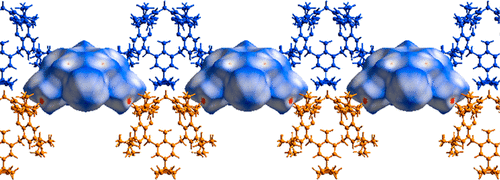
The high melting point in the synthetic description of calixarenes has been described as a common characteristic of these compounds. In order to explore the origin of the high thermal stability of calixarenes, a thermochemical and single-crystal X-ray diffraction study for solvent-free p-tert-butyl-calix[4]arene (PTBC4), p-tert-butyl-calix[5]arene (PTBC5), and p-tert-butyl-calix[6]arene (PTBC6) was performed, along with the analysis of the intermolecular interactions in the crystal phase through the Hirshfeld surface (HS) calculations. The experimental values obtained and trend of thermodynamic properties of temperature of fusion, as well as enthalpies of fusion and sublimation, are well supported by the single-crystal X-ray structure analysis. This dual combination allowed us to rationalize the chemical behavior of the calixarenes under study. The study of the intermolecular interactions in these calixarenes also includes the available crystallographic information for analogues PTBC4 and PTBC5 previously published in the literature. A comparative study is reported, together with the novel crystallographic structure of PTBC6. The synthetic procedure of a pure sample of PTBC6 is also described. Differential scanning calorimetry has shown through the melting point that the thermal stability of the crystals of calixarenes PTBC4, PTBC5, and PTBC6 is over 580 K. Comparison of the temperature of fusion of the corresponding oligomers reveals a macrocyclic effect, which can be attributed to the thermal stability of the three calixarenes. The values and trend for the enthalpies of sublimation are correctly supported by the crystal growth analysis.
中文翻译:

杯结构的热稳定性和大焓的融合和升华芳烃值的热焓的起源。
杯芳烃的合成描述中的高熔点已被描述为这些化合物的共同特征。为了探索杯芳烃,无溶剂热化学和单晶X射线衍射研究的高的热稳定性的原点p -叔丁基杯[4]芳烃(PTBC4),p -叔丁基-杯[5]芳烃(PTBC5)和对-叔进行了丁基丁基杯[6]芳烃(PTBC6),并通过Hirshfeld表面(HS)计算分析了结晶相中的分子间相互作用。单晶X射线结构分析很好地支持了获得的实验值和熔融温度的热力学性质趋势,以及熔融和升华的焓。这种双重组合使我们能够合理地研究所研究的杯芳烃的化学行为。在这些杯芳烃中的分子间相互作用的研究还包括以前文献中公开的类似物PTBC4和PTBC5的可用晶体学信息。进行了比较研究,以及PTBC6的新型晶体结构。还描述了PTBC6纯样品的合成程序。差示扫描量热法从熔点显示,杯芳烃PTBC4,PTBC5和PTBC6晶体的热稳定性超过580K。比较相应低聚物的熔融温度显示出大环效应,这可以归因于三个杯芳烃的热稳定性。晶体生长分析正确地支持了升华焓的值和趋势。
更新日期:2020-01-17
中文翻译:

杯结构的热稳定性和大焓的融合和升华芳烃值的热焓的起源。
杯芳烃的合成描述中的高熔点已被描述为这些化合物的共同特征。为了探索杯芳烃,无溶剂热化学和单晶X射线衍射研究的高的热稳定性的原点p -叔丁基杯[4]芳烃(PTBC4),p -叔丁基-杯[5]芳烃(PTBC5)和对-叔进行了丁基丁基杯[6]芳烃(PTBC6),并通过Hirshfeld表面(HS)计算分析了结晶相中的分子间相互作用。单晶X射线结构分析很好地支持了获得的实验值和熔融温度的热力学性质趋势,以及熔融和升华的焓。这种双重组合使我们能够合理地研究所研究的杯芳烃的化学行为。在这些杯芳烃中的分子间相互作用的研究还包括以前文献中公开的类似物PTBC4和PTBC5的可用晶体学信息。进行了比较研究,以及PTBC6的新型晶体结构。还描述了PTBC6纯样品的合成程序。差示扫描量热法从熔点显示,杯芳烃PTBC4,PTBC5和PTBC6晶体的热稳定性超过580K。比较相应低聚物的熔融温度显示出大环效应,这可以归因于三个杯芳烃的热稳定性。晶体生长分析正确地支持了升华焓的值和趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号