当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Straightforward Access to 2-Iodoindolizines via Iodine-Mediated Cyclization of 2-Pyridylallenes
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.oprd.9b00418 Thibaut Martinez 1 , Ismail Alahyen 1 , Gilles Lemière 1 , Virginie Mouriès-Mansuy 1 , Louis Fensterbank 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.oprd.9b00418 Thibaut Martinez 1 , Ismail Alahyen 1 , Gilles Lemière 1 , Virginie Mouriès-Mansuy 1 , Louis Fensterbank 1
Affiliation
|
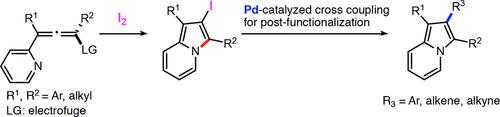
A metal-free access to 2-iodo-1,3-disubstituted indolizines has been developed. The proposed synthesis is relatively simple and efficient and involves the iodine-triggered 5-endo-trig cyclization of 2-pyridylallene precursors. While it can be conducted on a gram scale, the preparation of the precursors is straightforward and does not always require intermediate purifications. The obtained 2-iodoindolizines can be further functionalized through cross-coupling reactions.
中文翻译:

通过碘介导的2-吡啶基烯丙基环化反应,直接获得2-碘吲哚并
已经开发出无金属的2-碘-1,3-二取代的吲哚嗪类药物。拟议的合成相对简单和有效,涉及2-吡啶基丙二烯前体的碘触发的5-内-触发环化。虽然可以以克为单位进行,但前体的制备是直接的,并不总是需要中间纯化。所得的2-碘吲哚并嗪可以通过交叉偶联反应进一步官能化。
更新日期:2020-01-10
中文翻译:

通过碘介导的2-吡啶基烯丙基环化反应,直接获得2-碘吲哚并
已经开发出无金属的2-碘-1,3-二取代的吲哚嗪类药物。拟议的合成相对简单和有效,涉及2-吡啶基丙二烯前体的碘触发的5-内-触发环化。虽然可以以克为单位进行,但前体的制备是直接的,并不总是需要中间纯化。所得的2-碘吲哚并嗪可以通过交叉偶联反应进一步官能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号