当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed, para-Selective, Radical-Based Alkylation of Aromatic Ketones.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.orglett.9b04327 Jie Wang 1 , Yu-Bo Pang 1 , Na Tao 1 , Runsheng Zeng 1 , Yingsheng Zhao 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.orglett.9b04327 Jie Wang 1 , Yu-Bo Pang 1 , Na Tao 1 , Runsheng Zeng 1 , Yingsheng Zhao 1
Affiliation
|
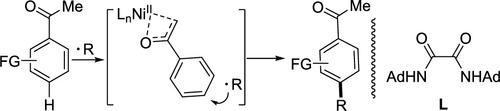
A direct, para-selective, radical-based alkylation of aromatic ketones with alkanes has been developed using a nickel catalyst with oxamide as the ligand. Acetophenones bearing electron-withdrawing substituents were functionalized directly with simple alkanes with high para-selectivity while acetophenones with electron-donating groups were mainly para-functionalized. A mechanistic study indicated that C-H bond activation of the aromatic ring may be the rate-determining step of the reaction.
中文翻译:

镍催化芳基酮的对位选择性,基于自由基的烷基化反应。
已经开发了使用以草酰胺为配体的镍催化剂,用烷烃对芳香族酮进行直接,对位,基于自由基的烷基化反应。带有吸电子取代基的苯乙酮可通过具有高对位选择性的简单烷烃直接官能化,而带有给电子基团的苯乙酮则主要被对官能化。机理研究表明,芳环的CH键活化可能是反应的速率决定步骤。
更新日期:2020-01-10
中文翻译:

镍催化芳基酮的对位选择性,基于自由基的烷基化反应。
已经开发了使用以草酰胺为配体的镍催化剂,用烷烃对芳香族酮进行直接,对位,基于自由基的烷基化反应。带有吸电子取代基的苯乙酮可通过具有高对位选择性的简单烷烃直接官能化,而带有给电子基团的苯乙酮则主要被对官能化。机理研究表明,芳环的CH键活化可能是反应的速率决定步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号