当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Azido-Enolonium Species in C-C and C-N Bond-Forming Coupling Reactions.
Organic Letters ( IF 5.2 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b03824 Atul A More 1 , Sourav K Santra 1 , Alex M Szpilman 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b03824 Atul A More 1 , Sourav K Santra 1 , Alex M Szpilman 1
Affiliation
|
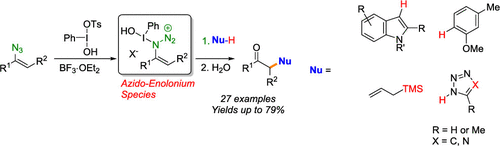
Vinyl azides react with boron trifluoride activated Koser's hypervalent iodine reagent to afford azido-enolonium species. These previously unknown azido-enolonium species react efficiently with aromatic compounds, allyltrimethylsilane, and azoles under mild conditions, with no need for a transition-metal catalyst, forming C-C and C-N bonds to give a variety of α-functionalized ketones. The intermediacy of the proposed azido-enolonium species is supported by spectroscopic studies.
中文翻译:

CC和CN键形成偶联反应中的叠氮En烯物种。
乙烯基叠氮化物与三氟化硼活化的Koser的高价碘试剂反应生成叠氮-烯类物质。这些先前未知的叠氮基-olo烯类物质在温和条件下与芳族化合物,烯丙基三甲基硅烷和唑类有效反应,无需过渡金属催化剂,形成CC和CN键即可生成各种α-官能化的酮。拟议的叠氮en烯物种的中间性得到了光谱学研究的支持。
更新日期:2020-01-10
中文翻译:

CC和CN键形成偶联反应中的叠氮En烯物种。
乙烯基叠氮化物与三氟化硼活化的Koser的高价碘试剂反应生成叠氮-烯类物质。这些先前未知的叠氮基-olo烯类物质在温和条件下与芳族化合物,烯丙基三甲基硅烷和唑类有效反应,无需过渡金属催化剂,形成CC和CN键即可生成各种α-官能化的酮。拟议的叠氮en烯物种的中间性得到了光谱学研究的支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号