当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of the Tricyclic Core of (-)-Callophycoic Acid A.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.joc.9b03114 Akihiro Sakama 1 , Rika Kameshima 1 , Yuko Motohashi 1 , Wataru Sumida 1 , Yuta Unno 1 , Keisuke Yoshida 1 , Akihiro Ogura 1 , Ken-Ichi Takao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.joc.9b03114 Akihiro Sakama 1 , Rika Kameshima 1 , Yuko Motohashi 1 , Wataru Sumida 1 , Yuta Unno 1 , Keisuke Yoshida 1 , Akihiro Ogura 1 , Ken-Ichi Takao 1
Affiliation
|
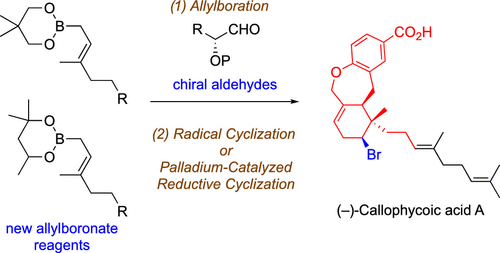
Two stereocontrolled routes to the tricyclic core of (-)-callophycoic acid A are described. Our synthetic strategy relied on stereoselective allylboration using a new allylboronate reagent to construct the all-carbon quaternary stereocenter in the core, followed by efficient radical cyclization or palladium-catalyzed reductive cyclization to form its multisubstituted cyclohexane ring. The tetrahydrooxepin ring was constructed by intramolecular etheration. This study provides the first method for the stereoselective synthesis of the characteristic tricyclic skeleton of callophycoic acids.
中文翻译:

立体选择性合成(-)-苯甲酸A.的三环核
描述了到(-)-苯甲酸A的三环核心的两个立体控制的路线。我们的合成策略依赖于立体选择性烯丙基硼化,使用一种新的烯丙基硼酸酯试剂在核心中构建全碳四元立体中心,然后进行有效的自由基环化或钯催化的还原环化,以形成其多取代的环己烷环。通过分子内醚化构建四氢氧杂环戊烯环。这项研究提供了第一种方法来立体选择性地合成愈伤组织酸的特征性三环骨架。
更新日期:2020-01-24
中文翻译:

立体选择性合成(-)-苯甲酸A.的三环核
描述了到(-)-苯甲酸A的三环核心的两个立体控制的路线。我们的合成策略依赖于立体选择性烯丙基硼化,使用一种新的烯丙基硼酸酯试剂在核心中构建全碳四元立体中心,然后进行有效的自由基环化或钯催化的还原环化,以形成其多取代的环己烷环。通过分子内醚化构建四氢氧杂环戊烯环。这项研究提供了第一种方法来立体选择性地合成愈伤组织酸的特征性三环骨架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号