当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sungeidines from a non-canonical enediyne biosynthetic pathway
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-10 , DOI: 10.1021/jacs.9b10086 Zhen Jie Low 1 , Guang-Lei Ma 1 , Hoa Thi Tran 1 , Yike Zou 2 , Juan Xiong 1, 3 , Limei Pang 1 , Selbi Nuryyeva 2 , Hong Ye 1 , Jin-Feng Hu 3 , K N Houk 2 , Zhao-Xun Liang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-10 , DOI: 10.1021/jacs.9b10086 Zhen Jie Low 1 , Guang-Lei Ma 1 , Hoa Thi Tran 1 , Yike Zou 2 , Juan Xiong 1, 3 , Limei Pang 1 , Selbi Nuryyeva 2 , Hong Ye 1 , Jin-Feng Hu 3 , K N Houk 2 , Zhao-Xun Liang 1
Affiliation
|
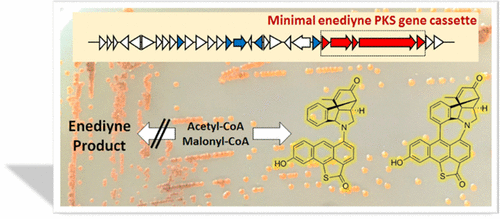
We report the genome-guided discovery of sungeidines, a class of microbial secondary metabolites with unique structural features. Despite evolutionary relationships with dynemicin-type enediynes, the sungeidines are produced by a biosynthetic gene cluster (BGC) that exhibits distinct differences from known enediyne BGCs. In accordance with a conserved minimal enediyne polyketide synthase (PKS) gene cassette and the 13C-isotope labeling results, the sungeidines are likely to be assembled from two octaketide chains following processing by oxygenases/oxidases and cyclases. An unusual activating sulfotransferase was also found to promote a 1, 2-trans-elimination reaction in sungeidine biosynthesis. We propose that the loss of genes, including a putative epoxidase gene, causes the divergence of the sungeidine pathway from other canonical enediyne pathways. The findings disclose the surprising evolvability of enediyne pathways and set the stage for characterizing the intriguing enzymatic steps in sungeidine biosynthesis.
中文翻译:

来自非经典烯二炔生物合成途径的双胍
我们报告了基因组引导下的双胞胎发现,这是一类具有独特结构特征的微生物次级代谢物。尽管与动力霉素型烯二炔存在进化关系,但双胍是由生物合成基因簇 (BGC) 产生的,该基因簇与已知的烯二炔 BGC 存在明显差异。根据保守的最小烯二炔聚酮化合物合酶 (PKS) 基因盒和 13C 同位素标记结果,在经过加氧酶/氧化酶和环化酶处理后,双炔化合物很可能由两条八酮链组装而成。还发现一种不寻常的活化磺基转移酶可促进双生地定生物合成中的 1, 2-反式消除反应。我们建议基因的丢失,包括一个推定的环氧化酶基因,导致双乙炔途径与其他经典烯二炔途径的分歧。这些发现揭示了烯二炔途径令人惊讶的可进化性,并为表征双庚啶生物合成中有趣的酶促步骤奠定了基础。
更新日期:2020-01-10
中文翻译:

来自非经典烯二炔生物合成途径的双胍
我们报告了基因组引导下的双胞胎发现,这是一类具有独特结构特征的微生物次级代谢物。尽管与动力霉素型烯二炔存在进化关系,但双胍是由生物合成基因簇 (BGC) 产生的,该基因簇与已知的烯二炔 BGC 存在明显差异。根据保守的最小烯二炔聚酮化合物合酶 (PKS) 基因盒和 13C 同位素标记结果,在经过加氧酶/氧化酶和环化酶处理后,双炔化合物很可能由两条八酮链组装而成。还发现一种不寻常的活化磺基转移酶可促进双生地定生物合成中的 1, 2-反式消除反应。我们建议基因的丢失,包括一个推定的环氧化酶基因,导致双乙炔途径与其他经典烯二炔途径的分歧。这些发现揭示了烯二炔途径令人惊讶的可进化性,并为表征双庚啶生物合成中有趣的酶促步骤奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号