当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A single point mutation converts GH84 O-GlcNAc hydrolases into phosphorylases. Experimental and theoretical evidence.
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b09655 David Teze 1, 2 , Joan Coines 3 , Lluís Raich 3 , Valentina Kalichuk 2 , Claude Solleux 2 , Charles Tellier 2 , Corinne André-Miral 2 , Birte Svensson 1 , Carme Rovira 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b09655 David Teze 1, 2 , Joan Coines 3 , Lluís Raich 3 , Valentina Kalichuk 2 , Claude Solleux 2 , Charles Tellier 2 , Corinne André-Miral 2 , Birte Svensson 1 , Carme Rovira 3, 4
Affiliation
|
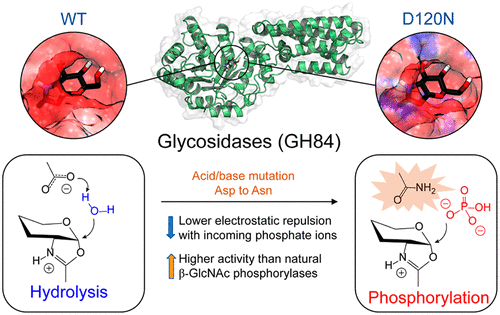
Glycoside hydrolases and phosphorylases are two major classes of enzymes responsible for the cleavage of glycosidic bonds. Here we show that two GH84 O-GlcNAcase enzymes can be converted to efficient phosphorylases by a single point mutation. Noteworthy, the mutated enzymes are over ten-fold more active than naturally occurring glucosaminide phosphorylases. We rationalize this novel transformation using molecular dynamics and QM/MM metadynamics methods, showing that the mutation changes the electrostatic potential at the active site and reduces the energy barrier for phosphorolysis by 10 kcal/mol. In addition, the simulations unambiguously reveal the nature of the intermediate as a glucose oxazolinium ion, clarifying the debate on the nature of such reaction intermediate in glycoside hydrolases operating via substrate-assisted catalysis.
中文翻译:

单点突变将 GH84 O-GlcNAc 水解酶转化为磷酸化酶。实验和理论证据。
糖苷水解酶和磷酸化酶是负责切割糖苷键的两大类酶。在这里,我们展示了两种 GH84 O-GlcNAcase 酶可以通过单点突变转化为有效的磷酸化酶。值得注意的是,突变酶的活性比天然存在的氨基葡萄糖磷酸化酶高十倍以上。我们使用分子动力学和 QM/MM 元动力学方法合理化了这种新的转化,表明突变改变了活性位点的静电势,并将磷酸解的能垒降低了 10 kcal/mol。此外,模拟明确地揭示了中间体作为葡萄糖恶唑啉离子的性质,澄清了关于通过底物辅助催化操作的糖苷水解酶中此类反应中间体性质的争论。
更新日期:2020-01-09
中文翻译:

单点突变将 GH84 O-GlcNAc 水解酶转化为磷酸化酶。实验和理论证据。
糖苷水解酶和磷酸化酶是负责切割糖苷键的两大类酶。在这里,我们展示了两种 GH84 O-GlcNAcase 酶可以通过单点突变转化为有效的磷酸化酶。值得注意的是,突变酶的活性比天然存在的氨基葡萄糖磷酸化酶高十倍以上。我们使用分子动力学和 QM/MM 元动力学方法合理化了这种新的转化,表明突变改变了活性位点的静电势,并将磷酸解的能垒降低了 10 kcal/mol。此外,模拟明确地揭示了中间体作为葡萄糖恶唑啉离子的性质,澄清了关于通过底物辅助催化操作的糖苷水解酶中此类反应中间体性质的争论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号