当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast dynamics and vibrational relaxation in six-coordinate heme proteins revealed by Femtosecond Stimulated Raman Spectroscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b10560 Carino Ferrante 1 , Giovanni Batignani , Emanuele Pontecorvo , Linda C Montemiglio , Marten H Vos 2 , Tullio Scopigno 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b10560 Carino Ferrante 1 , Giovanni Batignani , Emanuele Pontecorvo , Linda C Montemiglio , Marten H Vos 2 , Tullio Scopigno 1
Affiliation
|
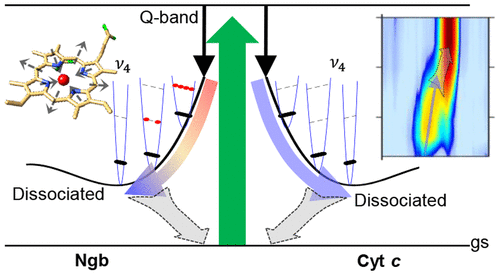
Identifying the structural rearrangements during photoinduced reactions is a fundamental challenge for understanding from a microscopic perspective the dynamics underlying the functional mechanisms of heme proteins. Here, femtosecond stimulated Raman spectroscopy is applied to follow the ultrafast evolution of two different proteins, each bearing a six-coordinate heme with two amino acid axial ligands. By exploiting the sensitivity of Raman spectra to the structural configuration, we investigate the effects of photolysis and the binding of amino acid residues in cytochrome c and neuroglobin. By comparing the system response for different time delays and Raman pump resonances, we show how detailed properties of atomic motions and energy redistribution can be unveiled. In particular, we demonstrate substantially faster energy flow from the dissociated heme to the protein moiety in cytochrome c, which we assign to the presence of covalent heme–protein bonds.
中文翻译:

飞秒受激拉曼光谱揭示六坐标血红素蛋白的超快动力学和振动弛豫
识别光诱导反应过程中的结构重排是从微观角度理解血红素蛋白功能机制背后的动力学的基本挑战。在这里,飞秒受激拉曼光谱用于跟踪两种不同蛋白质的超快进化,每种蛋白质都带有带有两个氨基酸轴向配体的六坐标血红素。通过利用拉曼光谱对结构构型的敏感性,我们研究了光解的影响以及细胞色素 c 和神经红蛋白中氨基酸残基的结合。通过比较不同时间延迟和拉曼泵共振的系统响应,我们展示了如何揭示原子运动和能量重新分布的详细特性。特别是,
更新日期:2020-01-09
中文翻译:

飞秒受激拉曼光谱揭示六坐标血红素蛋白的超快动力学和振动弛豫
识别光诱导反应过程中的结构重排是从微观角度理解血红素蛋白功能机制背后的动力学的基本挑战。在这里,飞秒受激拉曼光谱用于跟踪两种不同蛋白质的超快进化,每种蛋白质都带有带有两个氨基酸轴向配体的六坐标血红素。通过利用拉曼光谱对结构构型的敏感性,我们研究了光解的影响以及细胞色素 c 和神经红蛋白中氨基酸残基的结合。通过比较不同时间延迟和拉曼泵共振的系统响应,我们展示了如何揭示原子运动和能量重新分布的详细特性。特别是,











































 京公网安备 11010802027423号
京公网安备 11010802027423号