当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: an open-label, single arm, phase 2 trial.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41416-019-0698-9 Ali Belkouz 1 , Judith de Vos-Geelen 2 , Ron A A Mathôt 3 , Ferry A L M Eskens 4 , Thomas M van Gulik 5 , Martijn G H van Oijen 1 , Cornelis J A Punt 1 , Johanna W Wilmink 1 , Heinz-Josef Klümpen 1
British Journal of Cancer ( IF 6.4 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41416-019-0698-9 Ali Belkouz 1 , Judith de Vos-Geelen 2 , Ron A A Mathôt 3 , Ferry A L M Eskens 4 , Thomas M van Gulik 5 , Martijn G H van Oijen 1 , Cornelis J A Punt 1 , Johanna W Wilmink 1 , Heinz-Josef Klümpen 1
Affiliation
|
BACKGROUND
No standard treatment is available for advanced biliary tract cancer (BTC) after first-line therapy with gemcitabine plus cisplatin (GEMCIS). The objective of this study was to evaluate safety and anti-tumour activity of fluorouracil, leucovorin, irinotecan plus oxaliplatin (FOLFIRINOX) as salvage treatment in patients with previously treated advanced BTC.
METHODS
In this two-stage phase 2 study, patients with advanced BTC who had disease progression or unacceptable toxicity after ≥3 cycles of GEMCIS were eligible. Primary endpoints were safety and efficacy (defined as objective response rate, ORR). In stage one, ten patients were treated with FOLFIRINOX every 2 weeks. In stage two, an additional 20 patients were enrolled at a starting dose as defined in stage one, provided that in stage ≥1 objective response or ≥2 stable diseases were observed and ≤3 patients had serious adverse events (SAEs) within the first 6 weeks of treatment. Secondary endpoints were progression-free survival (PFS) and overall survival (OS).
RESULTS
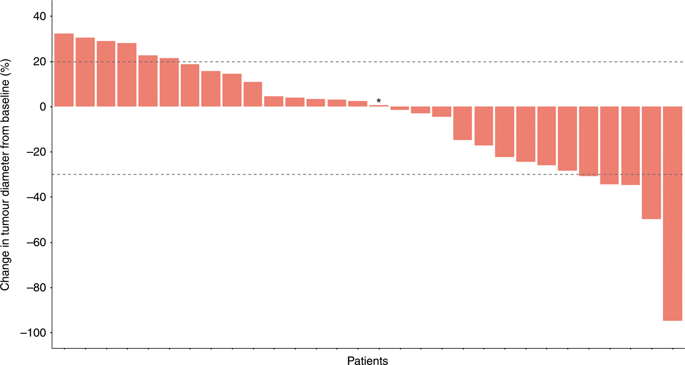
Forty patients were screened for eligibility and 30 patients were enrolled. In stage one, one patient had a partial response and five patients had stable disease. One patient had a SAE during the first 6 weeks of treatment, and five patients required a dose reduction due to adverse events. The most common grade 3-4 adverse events in stage one were neutropaenia, mucositis and diarrhoea. Stage two was initiated with FOLFIRINOX in an adapted dose. In stage two, grade 3-4 neutropaenia, diarrhoea, nausea and vomiting were the most common adverse events. The ORR, median PFS and OS in all patients were 10%, 6.2 and 10.7 months, respectively.
CONCLUSIONS
In patients with advanced BTC who progressed after or were intolerant to GEMCIS, FOLFIRINOX can be administered safely and could be considered as an option for salvage treatment in these patients.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov Identifier NCT02456714.
中文翻译:

FOLFIRINOX作为晚期胆道癌的挽救性治疗的功效和安全性:一项开放性单臂2期试验。
背景技术在吉西他滨联合顺铂(GEMCIS)一线治疗后,尚无用于晚期胆道癌(BTC)的标准治疗方法。这项研究的目的是评估氟尿嘧啶,亚叶酸钙,伊立替康加奥沙利铂(FOLFIRINOX)作为先前治疗的晚期BTC患者的挽救性治疗的安全性和抗肿瘤活性。方法在这项为期2期的2期研究中,≥3个GEMCIS周期后疾病进展或毒性不可接受的晚期BTC患者是合格的。主要终点是安全性和有效性(定义为客观缓解率,ORR)。在第一阶段,每2周用FOLFIRINOX治疗10名患者。在第二阶段,以第一阶段定义的起始剂量招募了另外20名患者,前提是在治疗的前6周内观察到≥1阶段的客观反应或≥2稳定的疾病,且≤3例患者出现严重不良事件(SAE)。次要终点是无进展生存期(PFS)和总体生存期(OS)。结果对40例患者进行了筛查,纳入30例患者。在第一阶段,一名患者部分缓解,五名患者病情稳定。一名患者在治疗的最初6周内患有SAE,五名患者由于不良事件而需要降低剂量。第一阶段最常见的3-4级不良反应是中性粒细胞减少,粘膜炎和腹泻。第二阶段以适应剂量的FOLFIRINOX开始。在第二阶段,最常见的不良事件为3-4级中性粒细胞减少,腹泻,恶心和呕吐。ORR 所有患者的中位PFS和OS分别为10%,6.2和10.7个月。结论在GEMCIS发生后或对GEMCIS不耐受的晚期BTC患者中,可以安全地使用FOLFIRINOX,可以考虑将其作为抢救治疗的选择。临床试验注册ClinicalTrials.gov标识符NCT02456714。
更新日期:2020-01-10
中文翻译:

FOLFIRINOX作为晚期胆道癌的挽救性治疗的功效和安全性:一项开放性单臂2期试验。
背景技术在吉西他滨联合顺铂(GEMCIS)一线治疗后,尚无用于晚期胆道癌(BTC)的标准治疗方法。这项研究的目的是评估氟尿嘧啶,亚叶酸钙,伊立替康加奥沙利铂(FOLFIRINOX)作为先前治疗的晚期BTC患者的挽救性治疗的安全性和抗肿瘤活性。方法在这项为期2期的2期研究中,≥3个GEMCIS周期后疾病进展或毒性不可接受的晚期BTC患者是合格的。主要终点是安全性和有效性(定义为客观缓解率,ORR)。在第一阶段,每2周用FOLFIRINOX治疗10名患者。在第二阶段,以第一阶段定义的起始剂量招募了另外20名患者,前提是在治疗的前6周内观察到≥1阶段的客观反应或≥2稳定的疾病,且≤3例患者出现严重不良事件(SAE)。次要终点是无进展生存期(PFS)和总体生存期(OS)。结果对40例患者进行了筛查,纳入30例患者。在第一阶段,一名患者部分缓解,五名患者病情稳定。一名患者在治疗的最初6周内患有SAE,五名患者由于不良事件而需要降低剂量。第一阶段最常见的3-4级不良反应是中性粒细胞减少,粘膜炎和腹泻。第二阶段以适应剂量的FOLFIRINOX开始。在第二阶段,最常见的不良事件为3-4级中性粒细胞减少,腹泻,恶心和呕吐。ORR 所有患者的中位PFS和OS分别为10%,6.2和10.7个月。结论在GEMCIS发生后或对GEMCIS不耐受的晚期BTC患者中,可以安全地使用FOLFIRINOX,可以考虑将其作为抢救治疗的选择。临床试验注册ClinicalTrials.gov标识符NCT02456714。











































 京公网安备 11010802027423号
京公网安备 11010802027423号