当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Lanosterol in Organic Solvents and in Water–Alcohol Mixtures at 101.8 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00390 Ke Li 1 , Daniel Forciniti 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00390 Ke Li 1 , Daniel Forciniti 1
Affiliation
|
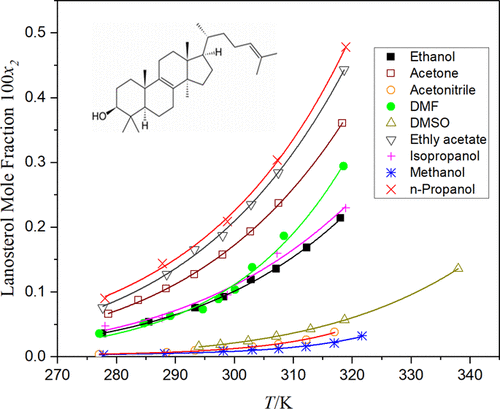
Lanosterol is a sterol derivative whose physicochemical properties are poorly understood. Pure lanosterol (>95%) was isolated from a crude product (54.6%) by a newly developed C18 reverse-phase high-performance liquid chromatography (HPLC) method. Purity and structure were confirmed by gas chromatography–mass spectrometry (GC–MS). The melting temperature and fusion enthalpy were determined to be 408.27 K and 23.61 kJ/mol, respectively. The solubility of lanosterol was measured in methanol, ethanol, isopropanol, n-propanol, acetonitrile, acetone, N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), ethyl acetate, water, water–methanol, water–isopropanol, and water–ethanol binary systems using a static equilibrium setup from 277.09 to 338.78 K. The solid phases were characterized by X-ray diffraction and by scanning differential calorimetry. The solubility of lanosterol increases with an increase in temperature. The mole fraction of lanosterol in organic solvents has a minimum of 3.0 × 10–5 in methanol at 277.78 K and a maximum of 0.0048 in n-propanol at 318.93 K. The solubilities of lanosterol in organic solvents were correlated by the modified Apelblat equation and by the Wilson, NRTL, and UNIQUAC models. Furthermore, the binary water–alcohol systems were correlated by the Apelblat–Jouyban–Acree and the van’t Hoff–Jouyban–Acree models.
中文翻译:

101.8 kPa下羊毛甾醇在有机溶剂和水-醇混合物中的溶解度
羊毛甾醇是一种其理化性质了解甚少的固醇衍生物。通过新开发的C18反相高效液相色谱(HPLC)方法从粗产物(54.6%)中分离出纯羊毛脂(> 95%)。纯度和结构通过气相色谱-质谱法(GC-MS)确认。测定的熔融温度和熔融焓分别为408.27K和23.61kJ / mol。测量了羊毛甾醇在甲醇,乙醇,异丙醇,正丙醇,乙腈,丙酮,N,N中的溶解度-二甲基甲酰胺(DMF),二甲基亚砜(DMSO),乙酸乙酯,水,水-甲醇,水-异丙醇和水-乙醇二元系统,使用277.09至338.78 K的静态平衡设置。固相的特征在于X-射线衍射和通过扫描差示热量法。羊毛甾醇的溶解度随着温度的升高而增加。羊毛甾醇在有机溶剂中的摩尔分数在277.78 K时在甲醇中最小为3.0×10 –5,在n中最大为0.0048。-丙醇在318.93 K处。羊毛甾醇在有机溶剂中的溶解度通过修正的Apelblat方程以及Wilson,NRTL和UNIQUAC模型进行关联。此外,二元水-酒精系统通过Apelblat-Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型进行关联。
更新日期:2020-01-10
中文翻译:

101.8 kPa下羊毛甾醇在有机溶剂和水-醇混合物中的溶解度
羊毛甾醇是一种其理化性质了解甚少的固醇衍生物。通过新开发的C18反相高效液相色谱(HPLC)方法从粗产物(54.6%)中分离出纯羊毛脂(> 95%)。纯度和结构通过气相色谱-质谱法(GC-MS)确认。测定的熔融温度和熔融焓分别为408.27K和23.61kJ / mol。测量了羊毛甾醇在甲醇,乙醇,异丙醇,正丙醇,乙腈,丙酮,N,N中的溶解度-二甲基甲酰胺(DMF),二甲基亚砜(DMSO),乙酸乙酯,水,水-甲醇,水-异丙醇和水-乙醇二元系统,使用277.09至338.78 K的静态平衡设置。固相的特征在于X-射线衍射和通过扫描差示热量法。羊毛甾醇的溶解度随着温度的升高而增加。羊毛甾醇在有机溶剂中的摩尔分数在277.78 K时在甲醇中最小为3.0×10 –5,在n中最大为0.0048。-丙醇在318.93 K处。羊毛甾醇在有机溶剂中的溶解度通过修正的Apelblat方程以及Wilson,NRTL和UNIQUAC模型进行关联。此外,二元水-酒精系统通过Apelblat-Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型进行关联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号