当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study on Isobaric Molar Heat Capacities of a Deep Eutectic Solvent: Choline Chloride + Ethylene Glycol
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00931 Chenyang Zhu 1 , Sa Xue 1 , Rana Ikram 1 , Xiangyang Liu 1 , Maogang He 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00931 Chenyang Zhu 1 , Sa Xue 1 , Rana Ikram 1 , Xiangyang Liu 1 , Maogang He 1
Affiliation
|
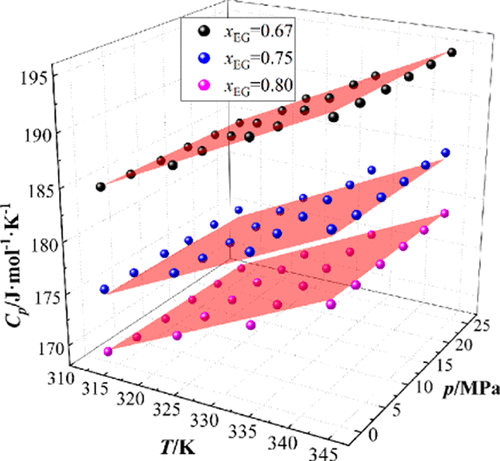
In this study, a flow calorimeter is used to measure the isobaric molar heat capacities (Cp) of one deep eutectic solvent (DES) containing choline chloride and ethylene glycol at different molar ratios. The experiment is performed at temperatures from 313 to 343 K and pressures up to 25 MPa, while the expanded uncertainties of temperature, pressure, molar fraction, and Cp are estimated to be lower than 0.02 K, 5.0 kPa, 1.4 × 10–4, and 1.28%, respectively. The results show that Cp change linearly with temperature, while it is almost constant with a variety of pressure. A quadratic relation between Cp and molar fraction of ethylene glycol is observed, which shows a decreasing tendency with the increase of molar fraction. A correlation for the Cp of the chosen DES and pure ethylene glycol is proposed for a better application of the data.
中文翻译:

深共晶溶剂:氯化胆碱+乙二醇的等压摩尔热容的实验研究
在这项研究中,使用量热仪来测量一种不同的摩尔比下含有氯化胆碱和乙二醇的深共熔溶剂(DES)的等压摩尔热容(C p)。实验是在313至343 K的温度和最高25 MPa的压力下进行的,而温度,压力,摩尔分数和C p的扩展不确定性估计低于0.02 K,5.0 kPa,1.4×10 –4,和1.28%。结果表明,C p随温度线性变化,而在各种压力下几乎恒定。C p之间的二次关系观察到乙二醇的摩尔分数,随摩尔分数的增加呈下降趋势。为了更好地应用数据,建议将所选DES与纯乙二醇的C p进行关联。
更新日期:2020-01-10
中文翻译:

深共晶溶剂:氯化胆碱+乙二醇的等压摩尔热容的实验研究
在这项研究中,使用量热仪来测量一种不同的摩尔比下含有氯化胆碱和乙二醇的深共熔溶剂(DES)的等压摩尔热容(C p)。实验是在313至343 K的温度和最高25 MPa的压力下进行的,而温度,压力,摩尔分数和C p的扩展不确定性估计低于0.02 K,5.0 kPa,1.4×10 –4,和1.28%。结果表明,C p随温度线性变化,而在各种压力下几乎恒定。C p之间的二次关系观察到乙二醇的摩尔分数,随摩尔分数的增加呈下降趋势。为了更好地应用数据,建议将所选DES与纯乙二醇的C p进行关联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号