Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A randomised phase II trial of hydroxychloroquine and imatinib versus imatinib alone for patients with chronic myeloid leukaemia in major cytogenetic response with residual disease.
Leukemia ( IF 12.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41375-019-0700-9 G A Horne 1 , J Stobo 2 , C Kelly 2 , A Mukhopadhyay 1 , A L Latif 1 , J Dixon-Hughes 2 , L McMahon 3 , P Cony-Makhoul 4 , J Byrne 5 , G Smith 6 , S Koschmieder 7 , T H BrÜmmendorf 7 , P Schafhausen 8 , P Gallipoli 9 , F Thomson 10 , W Cong 10 , R E Clark 11 , D Milojkovic 12 , G V Helgason 1 , L Foroni 13 , F E Nicolini 14 , T L Holyoake 1 , M Copland 1
Leukemia ( IF 12.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41375-019-0700-9 G A Horne 1 , J Stobo 2 , C Kelly 2 , A Mukhopadhyay 1 , A L Latif 1 , J Dixon-Hughes 2 , L McMahon 3 , P Cony-Makhoul 4 , J Byrne 5 , G Smith 6 , S Koschmieder 7 , T H BrÜmmendorf 7 , P Schafhausen 8 , P Gallipoli 9 , F Thomson 10 , W Cong 10 , R E Clark 11 , D Milojkovic 12 , G V Helgason 1 , L Foroni 13 , F E Nicolini 14 , T L Holyoake 1 , M Copland 1
Affiliation
|
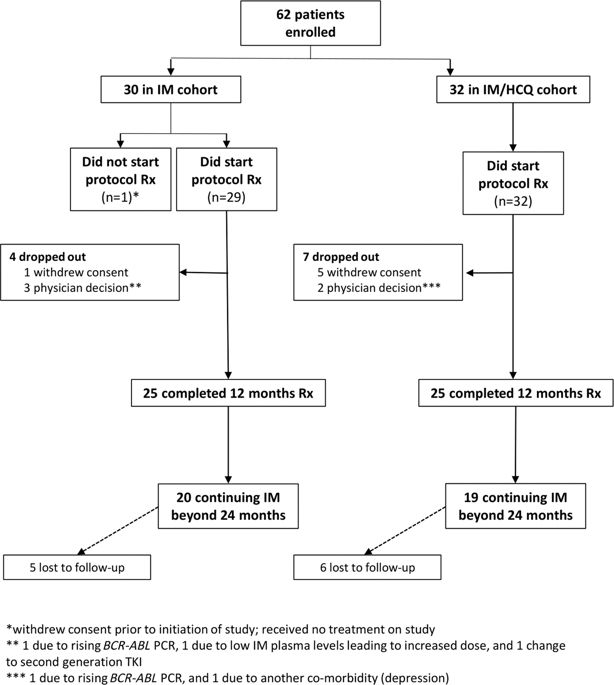
In chronic-phase chronic myeloid leukaemia (CP-CML), residual BCR-ABL1+ leukaemia stem cells are responsible for disease persistence despite TKI. Based on in vitro data, CHOICES (CHlorOquine and Imatinib Combination to Eliminate Stem cells) was an international, randomised phase II trial designed to study the safety and efficacy of imatinib (IM) and hydroxychloroquine (HCQ) compared with IM alone in CP-CML patients in major cytogenetic remission with residual disease detectable by qPCR. Sixty-two patients were randomly assigned to either arm. Treatment 'successes' was the primary end point, defined as ≥0.5 log reduction in 12-month qPCR level from trial entry. Selected secondary study end points were 24-month treatment 'successes', molecular response and progression at 12 and 24 months, comparison of IM levels, and achievement of blood HCQ levels >2000 ng/ml. At 12 months, there was no difference in 'success' rate (p = 0.58); MMR was achieved in 80% (IM) vs 92% (IM/HCQ) (p = 0.21). At 24 months, the 'success' rate was 20.8% higher with IM/HCQ (p = 0.059). No patients progressed. Seventeen serious adverse events, including four serious adverse reactions, were reported; diarrhoea occurred more frequently with combination. IM/HCQ is tolerable in CP-CML, with modest improvement in qPCR levels at 12 and 24 months, suggesting autophagy inhibition maybe of clinical value in CP-CML.
中文翻译:

一项随机 II 期试验,比较羟氯喹和伊马替尼与单用伊马替尼治疗慢性粒细胞白血病患者的主要细胞遗传学反应和残留疾病。
在慢性期慢性粒细胞白血病 (CP-CML) 中,尽管使用了 TKI,残留的 BCR-ABL1+ 白血病干细胞仍然是导致疾病持续存在的原因。 CHOICES(氯喹和伊马替尼组合消除干细胞)基于体外数据,是一项国际随机 II 期试验,旨在研究伊马替尼 (IM) 和羟氯喹 (HCQ) 与单独 IM 治疗 CP-CML 的安全性和有效性处于主要细胞遗传学缓解且可通过 qPCR 检测到残留疾病的患者。 62 名患者被随机分配到任一组。治疗“成功”是主要终点,定义为自试验开始后 12 个月 qPCR 水平降低≥0.5 个对数。选定的次要研究终点是 24 个月的治疗“成功”、12 个月和 24 个月的分子反应和进展、IM 水平的比较以及血液 HCQ 水平 > 2000 ng/ml 的实现。 12 个月时,“成功”率没有差异 (p = 0.58); MMR 的实现率为 80% (IM) vs 92% (IM/HCQ) (p = 0.21)。 24 个月时,IM/HCQ 的“成功”率提高了 20.8% (p = 0.059)。没有患者出现进展。报告了 17 例严重不良事件,其中 4 例严重不良反应;联合用药时腹泻发生的频率更高。 IM/HCQ 在 CP-CML 中是可耐受的,12 个月和 24 个月时 qPCR 水平略有改善,表明自噬抑制可能对 CP-CML 具有临床价值。
更新日期:2020-01-10
中文翻译:

一项随机 II 期试验,比较羟氯喹和伊马替尼与单用伊马替尼治疗慢性粒细胞白血病患者的主要细胞遗传学反应和残留疾病。
在慢性期慢性粒细胞白血病 (CP-CML) 中,尽管使用了 TKI,残留的 BCR-ABL1+ 白血病干细胞仍然是导致疾病持续存在的原因。 CHOICES(氯喹和伊马替尼组合消除干细胞)基于体外数据,是一项国际随机 II 期试验,旨在研究伊马替尼 (IM) 和羟氯喹 (HCQ) 与单独 IM 治疗 CP-CML 的安全性和有效性处于主要细胞遗传学缓解且可通过 qPCR 检测到残留疾病的患者。 62 名患者被随机分配到任一组。治疗“成功”是主要终点,定义为自试验开始后 12 个月 qPCR 水平降低≥0.5 个对数。选定的次要研究终点是 24 个月的治疗“成功”、12 个月和 24 个月的分子反应和进展、IM 水平的比较以及血液 HCQ 水平 > 2000 ng/ml 的实现。 12 个月时,“成功”率没有差异 (p = 0.58); MMR 的实现率为 80% (IM) vs 92% (IM/HCQ) (p = 0.21)。 24 个月时,IM/HCQ 的“成功”率提高了 20.8% (p = 0.059)。没有患者出现进展。报告了 17 例严重不良事件,其中 4 例严重不良反应;联合用药时腹泻发生的频率更高。 IM/HCQ 在 CP-CML 中是可耐受的,12 个月和 24 个月时 qPCR 水平略有改善,表明自噬抑制可能对 CP-CML 具有临床价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号