当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enriching lithium and separating lithium to magnesium from sulfate type salt lake brine
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105247 Xuheng Liu , Maoli Zhong , Xingyu Chen , Jiangtao Li , Lihua He , Zhongwei Zhao
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.hydromet.2020.105247 Xuheng Liu , Maoli Zhong , Xingyu Chen , Jiangtao Li , Lihua He , Zhongwei Zhao
|
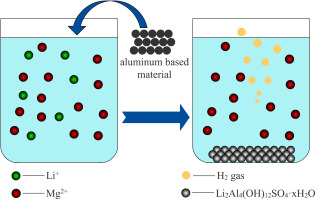
Abstract Extraction of lithium from salt lake brine has become a research highlights due to the rapid development of lithium ion battery. There are abundant lithium resources in sulfate type salt lake in China. In this work, the process of enriching lithium and separating lithium to magnesium from simulated sulfate type brine was carried out based on the reaction of Al/Na2SO4 composite with brine. The results show that Al/Na2SO4 composite can be used to enrich lithium from Li2SO4 solution in the form of Li2Al4(OH)12SO4·xH2O and the lithium precipitation efficiency reaches 89.2% under the optimal conditions. The existence of magnesium in solution is adverse to the precipitation process of lithium. The coating of compact Mg Al hydrotalcite on the surface of aluminum based material hinders the reaction of Al/Na2SO4 composite with brine and the lithium precipitation efficiency decreases to 54.7% when the Mg/Li mass ratio in solution is 20:1. However, the Mg/Li mass ratio in precipitate is less than 0.3 under the optimal conditions. The results are beneficial for the enriching lithium and separating lithium to magnesium from sulfate type brine.
中文翻译:

从硫酸盐型盐湖卤水中富锂分离锂镁
摘要 随着锂离子电池的快速发展,从盐湖卤水中提锂成为研究热点。我国硫酸盐型盐湖锂资源丰富。本工作以Al/Na2SO4复合物与卤水反应为基础,开展了从模拟硫酸盐型卤水中富锂分离锂成镁的过程。结果表明,Al/Na2SO4复合材料可以从Li2SO4溶液中以Li2Al4(OH)12SO4·xH2O的形式富集锂,在最佳条件下析锂效率达到89.2%。溶液中镁的存在不利于锂的沉淀过程。铝基材料表面致密的Mg Al水滑石涂层阻碍了Al/Na2SO4复合物与盐水的反应,当溶液中Mg/Li质量比为20:1时,析锂效率降低至54.7%。然而,在最佳条件下,沉淀物中的 Mg/Li 质量比小于 0.3。研究结果有利于从硫酸盐型卤水中富集锂和分离锂成镁。
更新日期:2020-03-01
中文翻译:

从硫酸盐型盐湖卤水中富锂分离锂镁
摘要 随着锂离子电池的快速发展,从盐湖卤水中提锂成为研究热点。我国硫酸盐型盐湖锂资源丰富。本工作以Al/Na2SO4复合物与卤水反应为基础,开展了从模拟硫酸盐型卤水中富锂分离锂成镁的过程。结果表明,Al/Na2SO4复合材料可以从Li2SO4溶液中以Li2Al4(OH)12SO4·xH2O的形式富集锂,在最佳条件下析锂效率达到89.2%。溶液中镁的存在不利于锂的沉淀过程。铝基材料表面致密的Mg Al水滑石涂层阻碍了Al/Na2SO4复合物与盐水的反应,当溶液中Mg/Li质量比为20:1时,析锂效率降低至54.7%。然而,在最佳条件下,沉淀物中的 Mg/Li 质量比小于 0.3。研究结果有利于从硫酸盐型卤水中富集锂和分离锂成镁。











































 京公网安备 11010802027423号
京公网安备 11010802027423号