当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxadiazolylthiazoles as novel and selective antifungal agents.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.ejmech.2020.112046 Mohamed Hagras 1 , Ehab A Salama 2 , Ahmed M Sayed 1 , Nader S Abutaleb 2 , Ahmed Kotb 1 , Mohamed N Seleem 3 , Abdelrahman S Mayhoub 4
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.ejmech.2020.112046 Mohamed Hagras 1 , Ehab A Salama 2 , Ahmed M Sayed 1 , Nader S Abutaleb 2 , Ahmed Kotb 1 , Mohamed N Seleem 3 , Abdelrahman S Mayhoub 4
Affiliation
|
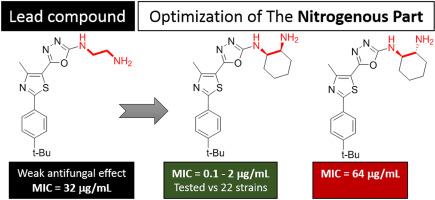
Studying the structure-activity relationships (SAR) of oxadiazolylthiazole antibiotics unexpectedly led us to identify ethylenediamine- and propylenediamine-analogs as potential antimycotic novel lead structures. Replacement of the ethylenediamine moiety for the lead compound 7 with cis-diaminocyclohexyl group (compound 18) significantly enhanced the antifungal activity. In addition to the high safety margin of 18 against mammalian cells, it showed highly selective broad-spectrum activity against fungal cells without inhibiting the human normal microbiota. The antifungal activity of 18 was investigated against 20 drug-resistant clinically important fungi, including Candida species, Cryptococcus, and Aspergillus fumigatus strains. In addition to the low MIC values that mostly ranged between 0.125 and 2.0 μg/mL, compound 18 outperformed fluconazole in disrupting mature Candida biofilm.
中文翻译:

恶二唑基噻唑类作为新型和选择性的抗真菌剂。
研究恶二唑基噻唑抗生素的构效关系(SAR)出乎意料地使我们发现乙二胺和丙二胺类似物是潜在的抗真菌药新的铅结构。用顺式-二氨基环己基(化合物18)代替前导化合物7的乙二胺部分显着增强了抗真菌活性。除了18对哺乳动物细胞的高安全系数外,它还显示出对真菌细胞的高选择性广谱活性,而没有抑制人类正常微生物群。研究了18种抗真菌活性对20种耐药的临床上重要的真菌,包括念珠菌,隐球菌和烟曲霉菌株。除了低MIC值(通常在0.125和2.0μg/ mL之间)之外,
更新日期:2020-01-10
中文翻译:

恶二唑基噻唑类作为新型和选择性的抗真菌剂。
研究恶二唑基噻唑抗生素的构效关系(SAR)出乎意料地使我们发现乙二胺和丙二胺类似物是潜在的抗真菌药新的铅结构。用顺式-二氨基环己基(化合物18)代替前导化合物7的乙二胺部分显着增强了抗真菌活性。除了18对哺乳动物细胞的高安全系数外,它还显示出对真菌细胞的高选择性广谱活性,而没有抑制人类正常微生物群。研究了18种抗真菌活性对20种耐药的临床上重要的真菌,包括念珠菌,隐球菌和烟曲霉菌株。除了低MIC值(通常在0.125和2.0μg/ mL之间)之外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号