Tetrahedron ( IF 2.1 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.tet.2020.130926 Xingrui Luo , Santhosh Kumar Pailla , Fei Gao , Xing Zheng , Ruowen Wang
|
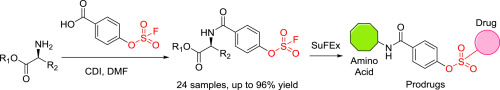
Sulfur (VI) Fluoride Exchange (SuFEx) chemistry is proposed as a new generation of click chemistry with potential in drug discovery and biological study. Herein we report a simple and convenient approach to synthesize amino acid derivatives functionalized with aryl fluorosulfonyl group from a simple building block. Promoted by 1,1′-Carbonyldiimidazole (CDI), methyl protected amino acids and other amines were efficiently functionalized with SO2F by the reaction with 4-((fluorosulfonyl)oxy)benzoic acid giving fluorosulfonylated amides (FSAs) as products. We also demonstrated that FSAs are useful building blocks in drug discovery. The conjugation of FSA with pharmaceutical phenols by SuFEx efficiently introduced amino acid moiety into target molecule, providing a series of prodrugs with diverse property.
中文翻译:

通过SuFEx化学方法用氟代硫酸芳基酯对氨基酸进行功能化以构建前药
硫(VI)氟化物交换(SuFEx)化学被认为是新一代点击化学,在药物发现和生物学研究中具有潜力。在本文中,我们报告了一种简单便捷的方法,可以从一个简单的结构单元中合成被芳基氟磺酰基官能化的氨基酸衍生物。在1,1'-羰基二咪唑(CDI)的促进下,甲基保护的氨基酸和其他胺通过与4-((氟磺酰基)氧基)苯甲酸反应,以SO 2 F有效地官能化,得到氟磺酰化酰胺(FSA)作为产物。我们还证明了FSA是药物发现中有用的基础。SuFEx将FSA与药用酚结合,可将氨基酸部分有效地引入靶分子,从而提供了一系列具有多种特性的前药。









































 京公网安备 11010802027423号
京公网安备 11010802027423号