当前位置:
X-MOL 学术
›
Mol. Omics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transcriptome profiling of hiPSC-derived LSECs with nanoCAGE.
Molecular Omics ( IF 3.0 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9mo00135b Mathieu Danoy 1 , Stéphane Poulain , Yuta Koui , Yannick Tauran , Benedikt Scheidecker , Taketomo Kido , Atsushi Miyajima , Yasuyuki Sakai , Charles Plessy , Eric Leclerc
Molecular Omics ( IF 3.0 ) Pub Date : 2020-01-28 , DOI: 10.1039/c9mo00135b Mathieu Danoy 1 , Stéphane Poulain , Yuta Koui , Yannick Tauran , Benedikt Scheidecker , Taketomo Kido , Atsushi Miyajima , Yasuyuki Sakai , Charles Plessy , Eric Leclerc
Affiliation
|
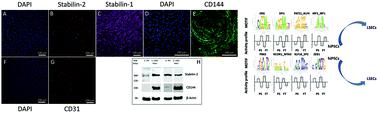
Liver Sinusoidal Endothelial Cells (LSECs) are an important component of the liver as they compose the microvasculature which allows the supply of oxygen, blood, and nutrients. However, maintenance of these cells in vitro remains challenging as they tend to rapidly lose some of their characteristics such as fenestration or as their immortalized counterparts present poor characteristics. In this work, human induced pluripotent stem cells (hiPSCs) have been differentiated toward an LSEC phenotype. After differentiation, the RNA quantification allowed demonstration of high expression of specific vascular markers (CD31, CD144, and STAB2). Immunostaining performed on the cells was found to be positive for both Stabilin-1 and Stabilin-2. Whole transcriptome analysis performed with the nanoCAGE method further confirmed the overall vascular commitment of the cells. The gene expression profile revealed the upregulation of the APLN, LYVE1, VWF, ESAM and ANGPT2 genes while VEGFA appeared to be downregulated. Analysis of promoter motif activities highlighted several transcription factors (TFs) of interest in LSECs (IRF2, ERG, MEIS2, SPI1, IRF7, WRNIP1, HIC2, NFIX_NFIB, BATF, and PATZ1). Based on this investigation, we compiled the regulatory network involving the relevant TFs, their target genes as well as their related signaling pathways. The proposed hiPSC-derived LSEC model and its regulatory network were then confirmed by comparing the experimental data to primary human LSEC reference datasets. Thus, the presented model appears as a promising tool to generate more complex in vitro liver multi-cellular tissues.
中文翻译:

hiPSC衍生的LSEC与nanoCAGE的转录组分析。
肝窦窦内皮细胞(LSEC)是肝脏的重要组成部分,因为它们构成了微血管,可以提供氧气,血液和营养。然而,这些细胞在体外的维持仍然具有挑战性,因为它们往往会迅速丧失其某些特征,例如开窗或永生化的对应物表现出较差的特征。在这项工作中,人类诱导的多能干细胞(hiPSC)已分化为LSEC表型。分化后,RNA定量显示了特定血管标记(CD31,CD144和STAB2)的高表达。发现对细胞进行的免疫染色对于Stabilin-1和Stabilin-2均为阳性。用nanoCAGE方法进行的整个转录组分析进一步证实了细胞的总体血管功能。基因表达谱显示APLN,LYVE1,VWF,ESAM和ANGPT2基因上调,而VEGFA似乎下调。启动子基序活性的分析突出了LSEC中感兴趣的几个转录因子(TFs)(IRF2,ERG,MEIS2,SPI1,IRF7,WRNIP1,HIC2,NFIX_NFIB,BATF和PATZ1)。基于此调查,我们编译了涉及相关TF,其靶标基因及其相关信号通路的调控网络。然后,通过将实验数据与主要的人类LSEC参考数据集进行比较,证实了拟议的hiPSC衍生的LSEC模型及其监管网络。从而,
更新日期:2020-01-10
中文翻译:

hiPSC衍生的LSEC与nanoCAGE的转录组分析。
肝窦窦内皮细胞(LSEC)是肝脏的重要组成部分,因为它们构成了微血管,可以提供氧气,血液和营养。然而,这些细胞在体外的维持仍然具有挑战性,因为它们往往会迅速丧失其某些特征,例如开窗或永生化的对应物表现出较差的特征。在这项工作中,人类诱导的多能干细胞(hiPSC)已分化为LSEC表型。分化后,RNA定量显示了特定血管标记(CD31,CD144和STAB2)的高表达。发现对细胞进行的免疫染色对于Stabilin-1和Stabilin-2均为阳性。用nanoCAGE方法进行的整个转录组分析进一步证实了细胞的总体血管功能。基因表达谱显示APLN,LYVE1,VWF,ESAM和ANGPT2基因上调,而VEGFA似乎下调。启动子基序活性的分析突出了LSEC中感兴趣的几个转录因子(TFs)(IRF2,ERG,MEIS2,SPI1,IRF7,WRNIP1,HIC2,NFIX_NFIB,BATF和PATZ1)。基于此调查,我们编译了涉及相关TF,其靶标基因及其相关信号通路的调控网络。然后,通过将实验数据与主要的人类LSEC参考数据集进行比较,证实了拟议的hiPSC衍生的LSEC模型及其监管网络。从而,











































 京公网安备 11010802027423号
京公网安备 11010802027423号