当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
meta-Substituted benzenesulfonamide: a potent scaffold for the development of metallo-β-lactamase ImiS inhibitors
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9md00455f Ya Liu 1 , Cheng Chen 1 , Le-Yun Sun 1 , Han Gao 1 , Jian-Bin Zhen 1 , Ke-Wu Yang 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9md00455f Ya Liu 1 , Cheng Chen 1 , Le-Yun Sun 1 , Han Gao 1 , Jian-Bin Zhen 1 , Ke-Wu Yang 1
Affiliation
|
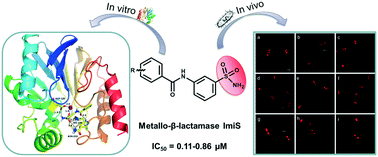
Metallo-β-lactamase (MβL) ImiS contributes to the emergence of carbapenem resistance. A potent scaffold, meta-substituted benzenesulfonamide, was constructed and assayed against MβLs. The twenty-one obtained molecules specifically inhibited ImiS (IC50 = 0.11–9.3 μM); 2g was found to be the best inhibitor (IC50 = 0.11 μM), and 1g and 2g exhibited partially mixed inhibition with Ki of 8.0 and 0.55 μM. The analysis of the structure–activity relationship revealed that the meta-substitutes improved the inhibitory activity of the inhibitors. Isothermal titration calorimetry (ITC) assays showed that 2g reversibly inhibited ImiS. The benzenesulfonamides exhibited synergistic antibacterial effects against E. coli BL21 (DE3) cells with ImiS, resulting in a 2–4-fold reduction in the MIC of imipenem and meropenem. Also, mouse experiments showed that 2g had synergistic efficacy with meropenem and significantly reduced the bacterial load in the spleen and liver after a single intraperitoneal dose. Tracing the ImiS in living E. coli cells by RS at a super-resolution level (3D-SIM) showed that the target was initially associated on the surface of the cells, then there was a high density of uniform localization distributed in the cytosol of cells, and it finally accumulated in the formation of inclusion bodies at the cell poles. Docking studies suggested that the sulfonamide group acted as a zinc-binding group to coordinate with Zn(II) and the residual amino acid within the CphA active center, tightly anchoring the inhibitor at the active site. This study provides a highly promising scaffold for the development of inhibitors of ImiS, even the B2 subclasses of MβLs.
中文翻译:

间位取代的苯磺酰胺:用于开发金属-β-内酰胺酶 ImiS 抑制剂的有效支架
金属-β-内酰胺酶 (MβL) ImiS 导致碳青霉烯类耐药性的出现。构建了一种有效的支架,即间位取代的苯磺酰胺,并针对 MβL 进行了分析。获得的 21 个分子特异性抑制 ImiS (IC 50 = 0.11–9.3 μM);发现2g是最好的抑制剂(IC 50 = 0.11 μM),1g和2g表现出部分混合抑制,K i分别为8.0和0.55 μM。构效关系分析表明,间位取代物提高了抑制剂的抑制活性。等温滴定量热法 (ITC) 测定表明2g可逆地抑制 ImiS。苯磺酰胺与 ImiS 对大肠杆菌BL21 (DE3) 细胞表现出协同抗菌作用,导致亚胺培南和美罗培南的 MIC 降低 2-4 倍。此外,小鼠实验表明,2g与美罗培南具有协同作用,单次腹腔注射后可显着降低脾脏和肝脏中的细菌负荷。通过超分辨率水平 (3D-SIM) 追踪活体大肠杆菌细胞中的 ImiS表明,目标最初与细胞表面相关,然后在细胞质中分布有高密度的均匀定位。细胞内,最终在细胞两极积累形成包涵体。对接研究表明,磺酰胺基团充当锌结合基团,与 Zn( II ) 和 CphA 活性中心内的残留氨基酸配位,将抑制剂紧密锚定在活性位点。这项研究为 ImiS 抑制剂的开发提供了一个非常有前景的支架,甚至是 MβL 的 B2 亚类。
更新日期:2020-02-27
中文翻译:

间位取代的苯磺酰胺:用于开发金属-β-内酰胺酶 ImiS 抑制剂的有效支架
金属-β-内酰胺酶 (MβL) ImiS 导致碳青霉烯类耐药性的出现。构建了一种有效的支架,即间位取代的苯磺酰胺,并针对 MβL 进行了分析。获得的 21 个分子特异性抑制 ImiS (IC 50 = 0.11–9.3 μM);发现2g是最好的抑制剂(IC 50 = 0.11 μM),1g和2g表现出部分混合抑制,K i分别为8.0和0.55 μM。构效关系分析表明,间位取代物提高了抑制剂的抑制活性。等温滴定量热法 (ITC) 测定表明2g可逆地抑制 ImiS。苯磺酰胺与 ImiS 对大肠杆菌BL21 (DE3) 细胞表现出协同抗菌作用,导致亚胺培南和美罗培南的 MIC 降低 2-4 倍。此外,小鼠实验表明,2g与美罗培南具有协同作用,单次腹腔注射后可显着降低脾脏和肝脏中的细菌负荷。通过超分辨率水平 (3D-SIM) 追踪活体大肠杆菌细胞中的 ImiS表明,目标最初与细胞表面相关,然后在细胞质中分布有高密度的均匀定位。细胞内,最终在细胞两极积累形成包涵体。对接研究表明,磺酰胺基团充当锌结合基团,与 Zn( II ) 和 CphA 活性中心内的残留氨基酸配位,将抑制剂紧密锚定在活性位点。这项研究为 ImiS 抑制剂的开发提供了一个非常有前景的支架,甚至是 MβL 的 B2 亚类。



























 京公网安备 11010802027423号
京公网安备 11010802027423号