当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of homogeneous plasmonic potency assay using gold nanoparticle immunocomplexes.
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jpba.2020.113101 Jin-Hee Han 1 , Fang Li 1 , Rico C Gunawan 1
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.4 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jpba.2020.113101 Jin-Hee Han 1 , Fang Li 1 , Rico C Gunawan 1
Affiliation
|
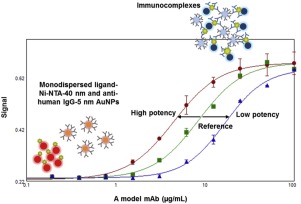
We evaluated the use of gold nanoparticles (AuNPs) platform in a homogenous assay for a potency measurement of a therapeutic monoclonal antibody (mAb). The recombinant human ligand protein to the therapeutic mAb was immobilized on AuNPs via functionalized self-assembled monolayers. Binding of the mAb to ligand lead to plasmonic signals that were detected faster in a homogeneous assay than the conventional enzyme-linked immunosorbent assay (ELISA). In this study, we demonstrated that the AuNP-based homogeneous plasmonic immunoassay (HPI) generated comparable potency values of a therapeutic mAb to a conventional binding ELISA in relatively shorter assay time and steps. Binding HPI can be potentially implemented as a potency assay for therapeutic mAbs in quality control laboratories.
中文翻译:

使用金纳米粒子免疫复合物的均质等离子体效能测定方法的发展。
我们评估了均相测定中金纳米颗粒(AuNPs)平台在治疗性单克隆抗体(mAb)效价测量中的使用。通过功能化的自组装单层将治疗性单抗的重组人配体蛋白固定在AuNPs上。mAb与配体的结合导致了等离子体信号,该信号在均相测定中比常规酶联免疫吸附测定(ELISA)更快。在这项研究中,我们证明了基于AuNP的均质等离子体免疫测定(HPI)在相对较短的测定时间和步骤中产生了与传统结合ELISA相当的治疗性mAb效能值。结合HPI可能在质量控制实验室中用作治疗性单克隆抗体的效价测定。
更新日期:2020-01-11
中文翻译:

使用金纳米粒子免疫复合物的均质等离子体效能测定方法的发展。
我们评估了均相测定中金纳米颗粒(AuNPs)平台在治疗性单克隆抗体(mAb)效价测量中的使用。通过功能化的自组装单层将治疗性单抗的重组人配体蛋白固定在AuNPs上。mAb与配体的结合导致了等离子体信号,该信号在均相测定中比常规酶联免疫吸附测定(ELISA)更快。在这项研究中,我们证明了基于AuNP的均质等离子体免疫测定(HPI)在相对较短的测定时间和步骤中产生了与传统结合ELISA相当的治疗性mAb效能值。结合HPI可能在质量控制实验室中用作治疗性单克隆抗体的效价测定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号