当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring‐Strain‐Promoted Ultrafast Diaryltetrazole–Alkyne Photoclick Reactions Triggered by Visible Light
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-01-22 , DOI: 10.1002/cptc.201900290 Shichao Jiang 1 , Xueting Wu 1 , Hui Liu 1 , Jiajie Deng 1 , Xiaocui Zhang 1 , Zhuojun Yao 1 , Yuanqin Zheng 1 , Bo Li 1 , Zhipeng Yu 1
ChemPhotoChem ( IF 3.0 ) Pub Date : 2020-01-22 , DOI: 10.1002/cptc.201900290 Shichao Jiang 1 , Xueting Wu 1 , Hui Liu 1 , Jiajie Deng 1 , Xiaocui Zhang 1 , Zhuojun Yao 1 , Yuanqin Zheng 1 , Bo Li 1 , Zhipeng Yu 1
Affiliation
|
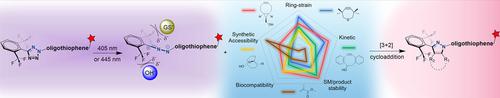
By exploiting the scope of dipolarophiles, we discovered that endo‐bicyclo[6.1.0]non‐4‐yn‐9‐ylmethanol (BCN) is an appropriate chemical reporter for the 2,5‐diaryltetrazole (DATet) based photoclick reaction, which proceeds via an ultrafast cycloaddition rate (∼105 M−1 s−1). Introduction of a 2,6‐(CF3)2phenyl moiety on the C5‐position of the tetrazole core brings a significant improvement of the chemical selectivity toward BCN without loss of reactivity. The high selectivity benefits from a combination of the steric and the electrostatic sheltering effect of a nitrile imine (NI) intermediate generated from photolysis of the DATet. In the absence of BCN, the oligothiophene moiety on the N2‐position of the tetrazole was found to induce self‐quenching of the NI intermediate instead of nucleophilic addition by 10 mM glutathione, demonstrating the fidelity of the DATet reagent. We also investigated the bioorthogonality of the DATet‐BCN photoclick chemistry for covalent bioconjugation toward BCN tags on proteins with visible‐light illumination.
中文翻译:

可见光触发的环应变促进的超快二芳基四唑-炔烃光点击反应
通过利用偶极亲和性的范围,我们发现内双环[6.1.0] non-4-yn-9-基甲醇(BCN)是基于2,5-二芳基四唑(DATet)的光点击反应的合适化学报告物,通过超快的环加成速率(〜10 5 M -1 s -1)进行。在C 5上引入2,6-(CF 3)2苯基部分四唑核的位置显着提高了对BCN的化学选择性,而又不降低反应活性。高选择性得益于DATet光解产生的腈亚胺(NI)中间体的空间和静电屏蔽作用。在不存在BCN的情况下,发现四唑N 2位上的寡噻吩部分可诱导NI中间体的自我猝灭,而不是10 mM谷胱甘肽的亲核加成,这证明了DATet试剂的保真度。我们还研究了DATet-BCN photoclick化学的生物正交性,该蛋白在可见光照射下与蛋白质上的BCN标签共价共轭。
更新日期:2020-01-22
中文翻译:

可见光触发的环应变促进的超快二芳基四唑-炔烃光点击反应
通过利用偶极亲和性的范围,我们发现内双环[6.1.0] non-4-yn-9-基甲醇(BCN)是基于2,5-二芳基四唑(DATet)的光点击反应的合适化学报告物,通过超快的环加成速率(〜10 5 M -1 s -1)进行。在C 5上引入2,6-(CF 3)2苯基部分四唑核的位置显着提高了对BCN的化学选择性,而又不降低反应活性。高选择性得益于DATet光解产生的腈亚胺(NI)中间体的空间和静电屏蔽作用。在不存在BCN的情况下,发现四唑N 2位上的寡噻吩部分可诱导NI中间体的自我猝灭,而不是10 mM谷胱甘肽的亲核加成,这证明了DATet试剂的保真度。我们还研究了DATet-BCN photoclick化学的生物正交性,该蛋白在可见光照射下与蛋白质上的BCN标签共价共轭。











































 京公网安备 11010802027423号
京公网安备 11010802027423号