当前位置:
X-MOL 学术
›
Microchem. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and optimization of a stability-indicating chromatographic method for verapamil hydrochloride and its impurities in tablets using an analytical quality by design (AQbD) approach
Microchemical Journal ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.microc.2020.104610 Camila dos Santos Moreira , Felipe Rebello Lourenço
Microchemical Journal ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.microc.2020.104610 Camila dos Santos Moreira , Felipe Rebello Lourenço
|
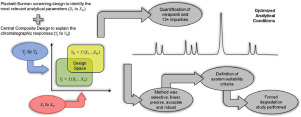
Abstract Development and optimization of analytical methods for quantifying verapamil hydrochloride in its twelve impurities is a challenge for analytical chemists and pharmacists. The aim of this paper was to apply the analytical quality by design (AQbD) and design of experiments (DoE) approaches in the development of a robust, low-cost, regulatory-flexible, fit for purpose high-performance liquid chromatography (HPLC) analytical method to determine VPM and its impurities. A Placket-Burman screening design was adopted to identify the most relevant analytical conditions affecting the chromatographic responses. In addition, a central composite optimization design was used to establish regression models explaining the chromatographic responses as functions of the buffer pH, concentration of ammonium hydroxide in mobile phase, and the injection volume of test solutions. Response surface methodology allowed for establishing the method operable design space, which ensures suitable method performance with respect to all chromatographic responses studied. Optimal chromatographic conditions were achieved using a gradient elution consisting of a mixture of 10 mM ammonium formate buffer pH and acetonitrile, with a flow rate of 0.7 mL/min, and a XSelect CSH C18 100 mm x 4.6 mm 3.5 µm chromatographic column. The optimized analytical method was selective, linear (320–480 µg/mL for VPM and 0.4–4.8 µg/mL for impurities), precise (0.5% and 1.0% for VPM and impurities, respectively), accurate (mean recovery of 101.0% and 102.5% for VPM and impurities, respectively), and robust. In addition, the application of the validated analytical method in forced degradation conditions allowed for studying the degradation profile of verapamil hydrochloride, particularly in acid and oxidative degradation conditions.
中文翻译:

使用分析质量源于设计 (AQbD) 方法开发和优化片剂中盐酸维拉帕米及其杂质的稳定性指示色谱方法
摘要 开发和优化盐酸维拉帕米十二种杂质的定量分析方法是分析化学家和药剂师面临的挑战。本文的目的是将分析质量源于设计 (AQbD) 和实验设计 (DoE) 方法应用于开发稳健、低成本、监管灵活、适用于目的的高效液相色谱 (HPLC)测定 VPM 及其杂质的分析方法。采用 Plaket-Burman 筛选设计来确定影响色谱响应的最相关分析条件。此外,使用中心复合优化设计建立回归模型,将色谱响应解释为缓冲液 pH、流动相中氢氧化铵浓度、和测试溶液的注入量。响应面方法允许建立方法可操作的设计空间,从而确保对于所研究的所有色谱响应具有合适的方法性能。使用由 10 mM 甲酸铵缓冲液 pH 值和乙腈的混合物组成的梯度洗脱获得最佳色谱条件,流速为 0.7 mL/min,以及 XSelect CSH C18 100 mm x 4.6 mm 3.5 µm 色谱柱。优化的分析方法具有选择性、线性(VPM 为 320–480 µg/mL,杂质为 0.4–4.8 µg/mL)、精确(VPM 和杂质分别为 0.5% 和 1.0%)、准确(平均回收率为 101.0%) VPM 和杂质分别为 102.5%),并且稳健。此外,
更新日期:2020-05-01
中文翻译:

使用分析质量源于设计 (AQbD) 方法开发和优化片剂中盐酸维拉帕米及其杂质的稳定性指示色谱方法
摘要 开发和优化盐酸维拉帕米十二种杂质的定量分析方法是分析化学家和药剂师面临的挑战。本文的目的是将分析质量源于设计 (AQbD) 和实验设计 (DoE) 方法应用于开发稳健、低成本、监管灵活、适用于目的的高效液相色谱 (HPLC)测定 VPM 及其杂质的分析方法。采用 Plaket-Burman 筛选设计来确定影响色谱响应的最相关分析条件。此外,使用中心复合优化设计建立回归模型,将色谱响应解释为缓冲液 pH、流动相中氢氧化铵浓度、和测试溶液的注入量。响应面方法允许建立方法可操作的设计空间,从而确保对于所研究的所有色谱响应具有合适的方法性能。使用由 10 mM 甲酸铵缓冲液 pH 值和乙腈的混合物组成的梯度洗脱获得最佳色谱条件,流速为 0.7 mL/min,以及 XSelect CSH C18 100 mm x 4.6 mm 3.5 µm 色谱柱。优化的分析方法具有选择性、线性(VPM 为 320–480 µg/mL,杂质为 0.4–4.8 µg/mL)、精确(VPM 和杂质分别为 0.5% 和 1.0%)、准确(平均回收率为 101.0%) VPM 和杂质分别为 102.5%),并且稳健。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号