当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulation of NCX1 by palmitoylation.
Cell Calcium ( IF 4 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.ceca.2019.102158 Caglar Gök 1 , William Fuller 1
Cell Calcium ( IF 4 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.ceca.2019.102158 Caglar Gök 1 , William Fuller 1
Affiliation
|
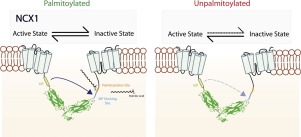
Palmitoylation (S-acylation) is the reversible conjugation of a fatty acid (usually C16 palmitate) to intracellular cysteine residues of proteins via a thioester linkage. Palmitoylation anchors intracellular regions of proteins to membranes because the palmitoylated cysteine is recruited to the lipid bilayer. NCX1 is palmitoylated at a single cysteine in its large regulatory intracellular loop. The presence of an amphipathic α-helix immediately adjacent to the NCX1 palmitoylation site is required for NCX1 palmitoylation. The NCX1 palmitoylation site is conserved through most metazoan phlya. Although palmitoylation does not regulate the normal forward or reverse ion transport modes of NCX1, NCX1 palmitoylation is required for its inactivation: sodium-dependent inactivation and inactivation by PIP2 depletion are significantly impaired for unpalmitoylatable NCX1. Here we review the role of palmitoylation in regulating NCX1 activity, and highlight future questions that must be addressed to fully understand the importance of this regulatory mechanism for sodium and calcium transport in cardiac muscle.
中文翻译:

棕榈酰化调控NCX1。
棕榈酰化(S-酰化)是脂肪酸(通常是C16棕榈酸酯)通过硫酯键与蛋白质的细胞内半胱氨酸残基的可逆结合。棕榈酰化将蛋白质的细胞内区域锚定在膜上,因为棕榈酰化的半胱氨酸被募集到脂质双层。NCX1在其较大的调节性细胞内环中的单个半胱氨酸上被棕榈酰化。NCX1棕榈酰化需要紧邻NCX1棕榈酰化位点的两亲性α-螺旋。NCX1的棕榈酰化位点在大多数后生动物的蝶突中均被保守。尽管棕榈酰化不能调节NCX1的正常正向或反向离子转运模式,但要使其失活,需要NCX1棕榈酰化:对于不可棕榈酰化的NCX1,钠依赖性的失活和PIP2消耗引起的失活显着受损。在这里,我们回顾了棕榈酰化在调节NCX1活性中的作用,并强调了必须解决的未来问题,以充分理解这种调节机制对心肌钠和钙转运的重要性。
更新日期:2020-01-09
中文翻译:

棕榈酰化调控NCX1。
棕榈酰化(S-酰化)是脂肪酸(通常是C16棕榈酸酯)通过硫酯键与蛋白质的细胞内半胱氨酸残基的可逆结合。棕榈酰化将蛋白质的细胞内区域锚定在膜上,因为棕榈酰化的半胱氨酸被募集到脂质双层。NCX1在其较大的调节性细胞内环中的单个半胱氨酸上被棕榈酰化。NCX1棕榈酰化需要紧邻NCX1棕榈酰化位点的两亲性α-螺旋。NCX1的棕榈酰化位点在大多数后生动物的蝶突中均被保守。尽管棕榈酰化不能调节NCX1的正常正向或反向离子转运模式,但要使其失活,需要NCX1棕榈酰化:对于不可棕榈酰化的NCX1,钠依赖性的失活和PIP2消耗引起的失活显着受损。在这里,我们回顾了棕榈酰化在调节NCX1活性中的作用,并强调了必须解决的未来问题,以充分理解这种调节机制对心肌钠和钙转运的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号