当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Examination of the Plausible Reaction Process for Stereoselective Synthesis of Hexapole Helicene via a Palladium-Catalyzed [2 + 2 + 2] Cyclotrimerization of [5]Helicenyl Aryne.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jpca.9b09533 Tomoka Hosokawa 1 , Toshio Asada 1, 2 , Ken Kamikawa 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jpca.9b09533 Tomoka Hosokawa 1 , Toshio Asada 1, 2 , Ken Kamikawa 1
Affiliation
|
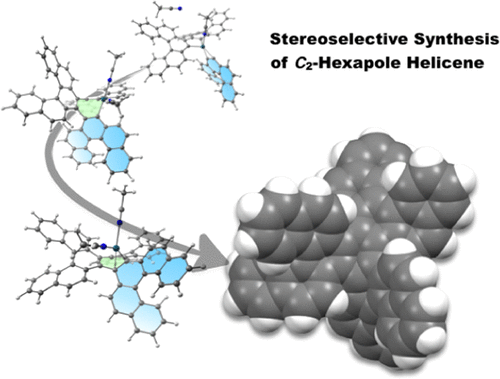
The reaction mechanisms of the plausible reaction process for the synthesis of hexapole helicene via a palladium-catalyzed [2 + 2 + 2] cyclotrimerization of [5]helicenyl aryne were examined using a theoretical approach. In a previous experimental study, this reaction selectively produced C2-symmetrical hexapole helicene, even though the D3-symmetrical structure is thermodynamically more stable. To clarify the mechanism underlying this reaction, density functional theory (DFT) and transition-state-theory calculations were used to evaluate the reaction profile and kinetic rate constants of the primary reactions. The thus obtained results suggest that the first step of the [2 + 2 + 2] cyclotrimerization is not a Diels-Alder reaction but an insertion of the helicenyl aryne into a metallacyclopentadiene. Subsequently, we clarified that the formation of the D3-symmetrical product is precluded by the high free-energy barrier of this reaction, while the C2-symmetrical product can be obtained at 300 K. Simulations of the time evolution of the molar fractions of the isomers were carried out based on the evaluated kinetic rate constants. The experimental result that the C2-symmetrical product is formed predominantly at 300 K was successfully reproduced in the simulations, while the isomerization into the more stable D3 hexapole helicene structure is predicted to occur at 400 K.
中文翻译:

通过钯催化的[5] Helicalenyl Aryne的[2 + 2 + 2]环三聚立体选择性合成六极杆螺旋烯的合理反应过程的理论研究。
使用理论方法,研究了通过[5] helicalenyl aryne的钯催化的[2 + 2 + 2]环三聚反应合成六极螺旋烯的合理反应过程的反应机理。在先前的实验研究中,即使D3对称结构在热力学上更稳定,该反应也会选择性地生成C2对称六极庚烯。为了阐明该反应的基础机理,使用密度泛函理论(DFT)和过渡态理论计算来评估主要反应的反应曲线和动力学速率常数。如此获得的结果表明,[2 + 2 + 2]环三聚的第一步不是狄尔斯-阿尔德反应,而是将烯丙基亚芳基插入金属环戊二烯中。后来,我们阐明,该反应的高自由能垒阻止了D3对称产物的形成,而C2对称产物可以在300 K下获得。对异构体摩尔分数的时间演化的模拟是根据评估的动力学速率常数进行。在模拟中成功再现了主要在300 K处形成C2对称产物的实验结果,而预计在400 K时会发生异构化为更稳定的D3六极螺旋结构。
更新日期:2020-01-23
中文翻译:

通过钯催化的[5] Helicalenyl Aryne的[2 + 2 + 2]环三聚立体选择性合成六极杆螺旋烯的合理反应过程的理论研究。
使用理论方法,研究了通过[5] helicalenyl aryne的钯催化的[2 + 2 + 2]环三聚反应合成六极螺旋烯的合理反应过程的反应机理。在先前的实验研究中,即使D3对称结构在热力学上更稳定,该反应也会选择性地生成C2对称六极庚烯。为了阐明该反应的基础机理,使用密度泛函理论(DFT)和过渡态理论计算来评估主要反应的反应曲线和动力学速率常数。如此获得的结果表明,[2 + 2 + 2]环三聚的第一步不是狄尔斯-阿尔德反应,而是将烯丙基亚芳基插入金属环戊二烯中。后来,我们阐明,该反应的高自由能垒阻止了D3对称产物的形成,而C2对称产物可以在300 K下获得。对异构体摩尔分数的时间演化的模拟是根据评估的动力学速率常数进行。在模拟中成功再现了主要在300 K处形成C2对称产物的实验结果,而预计在400 K时会发生异构化为更稳定的D3六极螺旋结构。










































 京公网安备 11010802027423号
京公网安备 11010802027423号