当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidase-Inspired Selective 2e/4e Reduction of Oxygen on Electron-Deficient Cu.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acsami.9b20920 Fei He 1 , Yan Zheng 1 , Huailin Fan 1 , Delong Ma 1 , Qifeng Chen 1 , Tao Wei 1 , Weibing Wu 1 , Dan Wu 2 , Xun Hu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-16 , DOI: 10.1021/acsami.9b20920 Fei He 1 , Yan Zheng 1 , Huailin Fan 1 , Delong Ma 1 , Qifeng Chen 1 , Tao Wei 1 , Weibing Wu 1 , Dan Wu 2 , Xun Hu 1
Affiliation
|
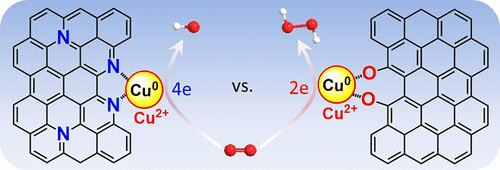
Development of low-cost and efficient (electro)catalysts with tunable 2e/4e oxygen reduction reaction (ORR) selectivity toward energy conversion, biomimetic catalysis, and biosensing has attracted growing interest. Herein, we reported that carbon nanohybrids with O- or N-coordinated Cu (Cu-OC or Cu-NC) showed superior activity for 2e and 4e electrocatalytic ORR with selectivities of 84.0% and 97.2%, respectively. Experimental evidence demonstrated that the strong electron-rich O-doped carbon in Cu-OC donated electrons to Cu2+, weakening the binding strength of H2O2 at Cu-O centers and facilitating the 2e ORR pathway for selective production of H2O2. However, the poor electron-donor ability of the N-doped carbon in Cu-NC made Cu-N sites more electron deficient due to the reduced electron transfer from N-doped carbon to Cu2+, promoting 4e ORR by enhancing adsorption of O2 and the ORR intermediates. The high 4e ORR activity of Cu-NC rendered its potential for application in a Zn-air battery and oxidase-mimicking activity for 3,3',5,5'-tetramethylbenzidine (TMB) and ascorbic acid (AA) oxidation. The maximal velocity (Vmax) of TMB and AA oxidation over Cu-NC was higher than some natural oxidases and noble-metal-based artificial enzymes. The lower activation energy for AA oxidation over Cu-NC resulted in a 263-fold higher oxidative rate than TMB, further prompting nonenzymatic sensing of AA by the competitive oxidation strategy.
中文翻译:

氧化酶激发的缺电子铜上氧的选择性2e / 4e还原。
具有可调节的2e / 4e氧还原反应(ORR)选择性的低成本和高效(电)催化剂对能量转换,仿生催化和生物传感的需求日益增长。本文中,我们报道了具有O或N配位的Cu(Cu-OC或Cu-NC)的碳纳米杂化物对2e和4e电催化ORR表现出优异的活性,选择性分别为84.0%和97.2%。实验证据表明,Cu-OC中强电子富集的O掺杂碳将电子提供给Cu2 +,削弱了H2O2在Cu-O中心的结合强度,并促进了2e ORR途径选择性生产H2O2。然而,由于减少了从N掺杂碳到Cu2 +的电子转移,Cu-NC中N掺杂碳的弱电子给体能力使Cu-N位更缺乏电子。通过增强O2和ORR中间体的吸附来促进4e ORR。Cu-NC的高4e ORR活性使其有潜力应用于锌空气电池中,并具有模仿3,3',5,5'-四甲基联苯胺(TMB)和抗坏血酸(AA)的氧化酶活性。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。-四甲基联苯胺(TMB)和抗坏血酸(AA)氧化。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。-四甲基联苯胺(TMB)和抗坏血酸(AA)氧化。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。
更新日期:2020-01-17
中文翻译:

氧化酶激发的缺电子铜上氧的选择性2e / 4e还原。
具有可调节的2e / 4e氧还原反应(ORR)选择性的低成本和高效(电)催化剂对能量转换,仿生催化和生物传感的需求日益增长。本文中,我们报道了具有O或N配位的Cu(Cu-OC或Cu-NC)的碳纳米杂化物对2e和4e电催化ORR表现出优异的活性,选择性分别为84.0%和97.2%。实验证据表明,Cu-OC中强电子富集的O掺杂碳将电子提供给Cu2 +,削弱了H2O2在Cu-O中心的结合强度,并促进了2e ORR途径选择性生产H2O2。然而,由于减少了从N掺杂碳到Cu2 +的电子转移,Cu-NC中N掺杂碳的弱电子给体能力使Cu-N位更缺乏电子。通过增强O2和ORR中间体的吸附来促进4e ORR。Cu-NC的高4e ORR活性使其有潜力应用于锌空气电池中,并具有模仿3,3',5,5'-四甲基联苯胺(TMB)和抗坏血酸(AA)的氧化酶活性。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。-四甲基联苯胺(TMB)和抗坏血酸(AA)氧化。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。-四甲基联苯胺(TMB)和抗坏血酸(AA)氧化。Cu-NC上TMB和AA氧化的最大速度(Vmax)高于某些天然氧化酶和基于贵金属的人工酶。与Cu-NC相比,AA氧化的活化能较低,因此氧化速率比TMB高263倍,这进一步促进了竞争性氧化策略对AA的非酶感测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号