当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chalcone-Based Pyridinium Salts and Their Diastereoselective Dearomatization To Access Bibridged Benzoazepines.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b04398 Le-Le Wang 1 , Hua-Bin Han 1 , Zhao-Hui Cui 1 , Jun-Wei Zhao 1 , Zhan-Wei Bu 1 , Qi-Lin Wang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b04398 Le-Le Wang 1 , Hua-Bin Han 1 , Zhao-Hui Cui 1 , Jun-Wei Zhao 1 , Zhan-Wei Bu 1 , Qi-Lin Wang 1
Affiliation
|
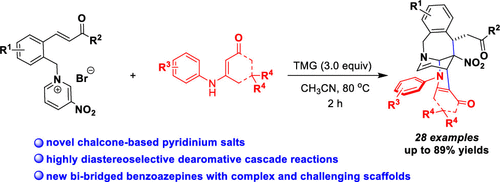
New chalcone-based pyridinium salts have been successfully exploited, which could smoothly participate in the highly diastereoselective dearomatization with binucleophilic enaminones by taking advantage of their multiple reactive sites to construct bibridged benzoazepines in up to 89% yields. The key to the success was the skillful and unprecedented C-3 functionalization of the new pyridinium salts. This work not only provides a kind of novel pyridinium salt synthon but also achieves the first C-3 functionalization of pyridinium salts to construct complex and challenging bibridged benzoazepines with high synthetic efficiency.
中文翻译:

查尔酮基吡啶鎓盐及其非对映选择性脱芳香化作用,以制得双桥苯并ze庚因。
新的基于查尔酮的吡啶鎓盐已被成功开发,可以利用它们的多个反应位点以高达89%的产率构建双桥苯并氮杂卓,从而顺利地参与双亲核性烯胺酮的高度非对映选择性脱芳香化作用。成功的关键是新型吡啶盐的C-3功能化和空前的熟练。这项工作不仅提供了一种新颖的吡啶鎓盐合成子,而且实现了吡啶鎓盐的第一个C-3官能化,从而以高合成效率构建了复杂且具有挑战性的双桥苯并氮杂。
更新日期:2020-01-09
中文翻译:

查尔酮基吡啶鎓盐及其非对映选择性脱芳香化作用,以制得双桥苯并ze庚因。
新的基于查尔酮的吡啶鎓盐已被成功开发,可以利用它们的多个反应位点以高达89%的产率构建双桥苯并氮杂卓,从而顺利地参与双亲核性烯胺酮的高度非对映选择性脱芳香化作用。成功的关键是新型吡啶盐的C-3功能化和空前的熟练。这项工作不仅提供了一种新颖的吡啶鎓盐合成子,而且实现了吡啶鎓盐的第一个C-3官能化,从而以高合成效率构建了复杂且具有挑战性的双桥苯并氮杂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号