当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh-Catalyzed Asymmetric Hydrogenation of Unsaturated Medium-Ring NH Lactams: Highly Enantioselective Synthesis of N-Unprotected 2,3-Dihydro-1,5-benzothiazepinones.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b04478 Congcong Yin 1 , Tao Yang 1 , Yingmin Pan 1 , Jialin Wen 1, 2 , Xumu Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.orglett.9b04478 Congcong Yin 1 , Tao Yang 1 , Yingmin Pan 1 , Jialin Wen 1, 2 , Xumu Zhang 1
Affiliation
|
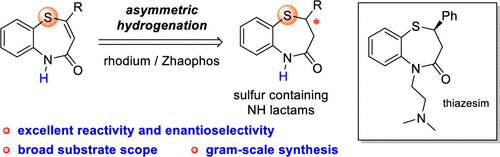
A straightforward method to prepare 1,5-benzothiazepines was reported. Catalyzed by a Rh/Zhaophos complex, unsaturated cyclic NH lactams with a medium-size ring were hydrogenated smoothly, giving remarkably high enantioselectivities. The sulfur atom in the substrates did not bring an inhibition which was observed with commercially available bisphosphine ligands. This method was successfully applied in the scale-up synthesis of (R)-(-)-thiazesim.
中文翻译:

Rh催化的不饱和中环NH内酰胺的不对称氢化:N-未保护的2,3-二氢-1,5-苯并噻唑酮的高度对映选择性合成。
据报道,一种简单的制备1,5-苯并硫氮杂s的方法。在Rh / Zhaophos配合物的催化下,具有中等大小的环的不饱和环状NH内酰胺被平滑地氢化,具有很高的对映选择性。底物中的硫原子没有带来抑制作用,这是通过市售双膦配体观察到的。该方法已成功应用于(R)-(-)-噻嗪酮的放大合成。
更新日期:2020-01-09
中文翻译:

Rh催化的不饱和中环NH内酰胺的不对称氢化:N-未保护的2,3-二氢-1,5-苯并噻唑酮的高度对映选择性合成。
据报道,一种简单的制备1,5-苯并硫氮杂s的方法。在Rh / Zhaophos配合物的催化下,具有中等大小的环的不饱和环状NH内酰胺被平滑地氢化,具有很高的对映选择性。底物中的硫原子没有带来抑制作用,这是通过市售双膦配体观察到的。该方法已成功应用于(R)-(-)-噻嗪酮的放大合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号