当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides.
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-01-09 , DOI: 10.3762/bjoc.16.8 Sabrina Müller 1 , Jannik Paulus 1 , Jochen Mattay 2 , Heiko Ihmels 3 , Veronica I Dodero 1 , Norbert Sewald 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-01-09 , DOI: 10.3762/bjoc.16.8 Sabrina Müller 1 , Jannik Paulus 1 , Jochen Mattay 2 , Heiko Ihmels 3 , Veronica I Dodero 1 , Norbert Sewald 1
Affiliation
|
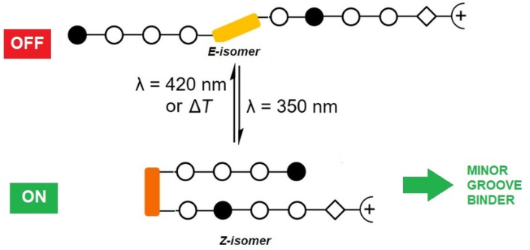
Azobenzenes are photoswitchable molecules capable of generating significant structural changes upon E-to-Z photoisomerization in peptides or small molecules, thereby controlling geometry and functionality. E-to-Z photoisomerization usually is achieved upon irradiation at 350 nm (π-π* transition), while the Z-to-E isomerization proceeds photochemically upon irradiation at >400 nm (n-π* transition) or thermally. Photoswitchable compounds have frequently been employed as modules, e.g., to control protein-DNA interactions. However, their use in conjunction with minor groove-binding imidazole/pyrrole (Im/Py) polyamides is yet unprecedented. Dervan-type Im/Py polyamides were equipped with an azobenzene unit, i.e., 3-(3-(aminomethyl)phenyl)azophenylacetic acid, as the linker between two Im/Py polyamide strands. Only the (Z)-azobenzene-containing polyamides bound to the minor groove of double-stranded DNA hairpins. Photoisomerization was exemplarily evaluated by 1H NMR experiments, while minor groove binding of the (Z)-azobenzene derivatives was proven by CD titration experiments. The resulting induced circular dichroism (ICD) bands of the bound ligands, together with the photometric determination of the dsDNA melting temperature, revealed a significant stabilization of the DNA upon association with the ligand. The (Z)-azobenzene acted as a building block inducing a reverse turn, which favored hydrogen bonds between the pyrrole/imidazole amide and the DNA bases. In contrast, the E-configured polyamides did not induce any ICD characteristic for minor groove binding. The incorporation of the photoswitchable azobenzene unit is a promising strategy to obtain photoswitchable Im/Py hairpin polyamides capable of interacting with the dsDNA minor groove only in the Z-configuration.
中文翻译:

咪唑/吡咯聚酰胺的光控DNA小沟相互作用。
偶氮苯是光可转换分子,能够在肽或小分子中进行E-Z光异构化后产生显着的结构变化,从而控制几何结构和功能。E-Z异构化通常是在350 nm(π-π*跃迁)下进行的,而Z-E异构化则是在> 400 nm(n-π*跃迁)下进行的,以光化学方式进行或通过热进行。光可开关化合物经常被用作模块,例如以控制蛋白质-DNA相互作用。但是,将它们与次要的结合沟的咪唑/吡咯(Im / Py)聚酰胺结合使用仍是前所未有的。Dervan型Im / Py聚酰胺配有偶氮苯单元,即3-(3-(氨基甲基)苯基)偶氮苯基乙酸,作为两条Im / Py聚酰胺链之间的连接基。仅含(Z)-偶氮苯的聚酰胺与双链DNA发夹的小沟结合。通过1 H NMR实验示例性地评估了光致异构化,同时通过CD滴定实验证明了(Z)-偶氮苯衍生物的微小凹槽结合。结合的配体产生的诱导的圆二色性(ICD)带,以及dsDNA熔解温度的光度测定,揭示了与配体缔合后DNA的显着稳定。(Z)-偶氮苯作为诱导逆转的结构单元,有利于吡咯/咪唑酰胺与DNA碱基之间的氢键。相反,E-构型的聚酰胺没有引起任何ICD特征,从而较小的沟槽结合。
更新日期:2020-01-09
中文翻译:

咪唑/吡咯聚酰胺的光控DNA小沟相互作用。
偶氮苯是光可转换分子,能够在肽或小分子中进行E-Z光异构化后产生显着的结构变化,从而控制几何结构和功能。E-Z异构化通常是在350 nm(π-π*跃迁)下进行的,而Z-E异构化则是在> 400 nm(n-π*跃迁)下进行的,以光化学方式进行或通过热进行。光可开关化合物经常被用作模块,例如以控制蛋白质-DNA相互作用。但是,将它们与次要的结合沟的咪唑/吡咯(Im / Py)聚酰胺结合使用仍是前所未有的。Dervan型Im / Py聚酰胺配有偶氮苯单元,即3-(3-(氨基甲基)苯基)偶氮苯基乙酸,作为两条Im / Py聚酰胺链之间的连接基。仅含(Z)-偶氮苯的聚酰胺与双链DNA发夹的小沟结合。通过1 H NMR实验示例性地评估了光致异构化,同时通过CD滴定实验证明了(Z)-偶氮苯衍生物的微小凹槽结合。结合的配体产生的诱导的圆二色性(ICD)带,以及dsDNA熔解温度的光度测定,揭示了与配体缔合后DNA的显着稳定。(Z)-偶氮苯作为诱导逆转的结构单元,有利于吡咯/咪唑酰胺与DNA碱基之间的氢键。相反,E-构型的聚酰胺没有引起任何ICD特征,从而较小的沟槽结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号