当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supported FexNiy catalysts for the co-activation of CO2 and small alkanes
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-1-9 , DOI: 10.1039/c9fd00130a Shaine Raseale 1, 2, 3, 4, 5 , Wijnand Marquart 1, 2, 3, 4, 5 , Kai Jeske 6, 7, 8 , Gonzalo Prieto 6, 7, 8 , Michael Claeys 1, 2, 3, 4, 5 , Nico Fischer 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-1-9 , DOI: 10.1039/c9fd00130a Shaine Raseale 1, 2, 3, 4, 5 , Wijnand Marquart 1, 2, 3, 4, 5 , Kai Jeske 6, 7, 8 , Gonzalo Prieto 6, 7, 8 , Michael Claeys 1, 2, 3, 4, 5 , Nico Fischer 1, 2, 3, 4, 5
Affiliation
|
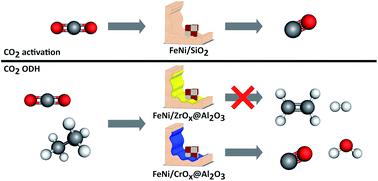
The effect of both the Fe : Ni ratio (5 to 1 : 1) and the relative Lewis acidity of a metal oxide support on catalytic activity, selectivity and stability was investigated in the CO2 mediated oxidative dehydrogenation of ethane (CO2-ODH). To avoid effects of varying pore sizes, shapes and volumes of the supports, chromia and zirconia overlayers were coated onto a common γ-Al2O3 carrier (CrOx@Al2O3 and ZrOx@Al2O3). Separately, oxidic FexNiy alloy precursor nanoparticles were prepared using a nonaqueous surfactant-free method and deposited by sonication onto the carrier. In comparison to previous studies in the field, this synthesis technique yields closely associated iron and nickel increasing the chances for alloy formation. During reduction, a mixture of a bcc and a fcc alloy phase was formed, with the content of bcc increasing with increasing iron content as predicted by the bulk phase diagram. Upon exposure to carbon dioxide at elevated temperatures, the bcc metallic phase is selectively oxidised to an inverse spinel structure via the dissociation of CO2. When exposed to CO2-ODH conditions, the bare ZrOx@Al2O3 support shows no activity. The presence of FeNi phases increases the conversion of ethane and CO2 marginally (<2%) but forms ethylene at high selectivity (SC2H4 > 80%). The CrOx@Al2O3 support shows some initial activity (XC2H6 < 5%) at very high ethylene selectivity (SC2H4 > 90%) but deactivates with time on stream. Comparison of the ethane and carbon dioxide conversions suggests that direct dehydrogenation rather than the oxidative pathway is taking place. When FeNi particles with the highest Fe content are added, the ethane conversion behavior hardly changes, but the CO2 conversion is increased now supporting the stoichiometric CO2-ODH reaction (SC2H4 > 95%). It is therefore evident that a tandem catalyst system between a reducible oxide carrier and the FeNi species is required. Increasing the Ni content results in an increase in activity and stability while changing the dominant reaction pathway to a combination of dry reforming, CO2-ODH and possibly the reverse Boudouard reaction, with the latter countering catalyst deactivation through carbon deposition.
中文翻译:

负载的FexNiy催化剂,用于CO2和小烷烃的共活化
在CO 2介导的乙烷氧化脱氢(CO 2 -ODH)中,研究了Fe:Ni比(5:1:1)和金属氧化物载体的相对路易斯酸度对催化活性,选择性和稳定性的影响。 。为了避免不同孔径,形状和支撑,氧化铬和氧化锆覆盖层的体积的效果进行涂覆到一个共同的γ-Al 2 ö 3载体(CRO X @Al 2 ö 3和ZrO X @Al 2 ö 3)。分别氧化Fe x Ni y使用无水表面活性剂方法制备合金前体纳米颗粒,并通过超声处理沉积到载体上。与该领域的先前研究相比,该合成技术可产生紧密相关的铁和镍,从而增加了形成合金的机会。在还原过程中,形成了bcc和fcc合金相的混合物,如体相图所示,bcc的含量随铁含量的增加而增加。在升高的温度下暴露于二氧化碳时,bcc金属相通过CO 2的解离选择性地氧化成倒尖晶石结构。暴露于CO 2 -ODH条件下,裸露的ZrO x @Al 2 O 3支持显示没有活动。FeNi相的存在会稍微提高乙烷和CO 2的转化率(<2%),但会以高选择性(S C 2 H 4 > 80%)形成乙烯。CrO x @Al 2 O 3载体在很高的乙烯选择性(S C 2 H 4)下显示出一些初始活性(X C 2 H 6 <5%)> 90%),但随着时间流逝而停用。乙烷和二氧化碳转化率的比较表明发生了直接脱氢而不是氧化途径。当加入具有最高Fe含量的FeNi颗粒中,乙烷转换行为几乎没有变化,但CO 2转化率提高现在支撑的化学计量CO 2 -ODH反应(小号c ^ 2 ^ h 4 > 95%)。因此,很明显,需要在可还原的氧化物载体和FeNi物质之间的串联催化剂体系。Ni含量的增加导致活性和稳定性的增加,同时将主要的反应途径改变为干重整,CO 2的组合-ODH和可能的反向Boudouard反应,后者通过碳沉积来阻止催化剂失活。
更新日期:2020-01-09
中文翻译:

负载的FexNiy催化剂,用于CO2和小烷烃的共活化
在CO 2介导的乙烷氧化脱氢(CO 2 -ODH)中,研究了Fe:Ni比(5:1:1)和金属氧化物载体的相对路易斯酸度对催化活性,选择性和稳定性的影响。 。为了避免不同孔径,形状和支撑,氧化铬和氧化锆覆盖层的体积的效果进行涂覆到一个共同的γ-Al 2 ö 3载体(CRO X @Al 2 ö 3和ZrO X @Al 2 ö 3)。分别氧化Fe x Ni y使用无水表面活性剂方法制备合金前体纳米颗粒,并通过超声处理沉积到载体上。与该领域的先前研究相比,该合成技术可产生紧密相关的铁和镍,从而增加了形成合金的机会。在还原过程中,形成了bcc和fcc合金相的混合物,如体相图所示,bcc的含量随铁含量的增加而增加。在升高的温度下暴露于二氧化碳时,bcc金属相通过CO 2的解离选择性地氧化成倒尖晶石结构。暴露于CO 2 -ODH条件下,裸露的ZrO x @Al 2 O 3支持显示没有活动。FeNi相的存在会稍微提高乙烷和CO 2的转化率(<2%),但会以高选择性(S C 2 H 4 > 80%)形成乙烯。CrO x @Al 2 O 3载体在很高的乙烯选择性(S C 2 H 4)下显示出一些初始活性(X C 2 H 6 <5%)> 90%),但随着时间流逝而停用。乙烷和二氧化碳转化率的比较表明发生了直接脱氢而不是氧化途径。当加入具有最高Fe含量的FeNi颗粒中,乙烷转换行为几乎没有变化,但CO 2转化率提高现在支撑的化学计量CO 2 -ODH反应(小号c ^ 2 ^ h 4 > 95%)。因此,很明显,需要在可还原的氧化物载体和FeNi物质之间的串联催化剂体系。Ni含量的增加导致活性和稳定性的增加,同时将主要的反应途径改变为干重整,CO 2的组合-ODH和可能的反向Boudouard反应,后者通过碳沉积来阻止催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号