当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile approach to alkaloid-like 6/6/5/5-tetracyclic spiroheterocycles via 1,3-dipolar cycloaddition reaction of fused 1H-pyrrole-2,3-diones with nitrones
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.tetlet.2020.151595 Ekaterina E. Stepanova , Maksim V. Dmitriev , Andrey N. Maslivets
中文翻译:

通过1 H-吡咯-2,3-二酮与硝酮的稠合反应的1,3-偶极环加成反应,轻松地制备生物碱样6/6/5 / 5-四环螺杂环
更新日期:2020-01-09
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.tetlet.2020.151595 Ekaterina E. Stepanova , Maksim V. Dmitriev , Andrey N. Maslivets
|
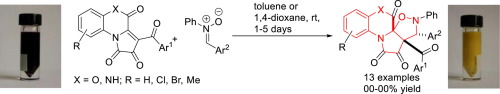
A facile synthetic approach towards 6/6/5/5-tetracyclic spiroheterocycles has been developed from the highly diastereoselective 1,3-dipolar cycloaddition reaction of [e]-fused 1H-pyrrole-2,3-diones with nitrones. The described novel heterocyclic systems are heteroanalogs of cytotoxic alkaloids, kibalaurifoline and gitingensine. The developed reaction represents the first example of involvement of 1H-pyrrole-2,3-diones fused at [e]-side in a 1,3-dipolar cycloaddition reaction.
中文翻译:

通过1 H-吡咯-2,3-二酮与硝酮的稠合反应的1,3-偶极环加成反应,轻松地制备生物碱样6/6/5 / 5-四环螺杂环
从[ e ]稠合的1 H-吡咯-2,3-二酮与硝酮的高度非对映选择性1,3-偶极环加成反应,已经开发出一种简便的合成方法,用于合成6/6/5 / 5-四环螺杂环。所描述的新型杂环系统是细胞毒性生物碱,奇巴拉妥林和gitingensine的杂类似物。发达的反应代表了1 H-吡咯-2,3-二酮在[ e ]侧融合在1,3-偶极环加成反应中的第一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号