Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.jfluchem.2020.109451 Wenchao Ye , Chuanfa Ni , Jinbo Hu
|
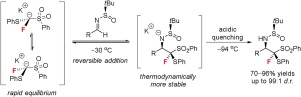
A diastereoselective nucleophilic monofluoromethylation of tert-butanesulfinyl aldimines with α-fluoro-α-phenylthio-α-phenylsulfonylmethane (FTSM) as the reagent has been developed, which affords α-monofluoromethyl amines in good to excellent yields with excellent stereocontrol on both the monofluoromethylated carbon and the neighboring sulfonyl-bearing fluorinated carbon. The racemic α-fluoro-α-phenylthio-α-phenylsulfonylmethide anions undergo a dynamic thermodynamic resolution due to the reversibility of their addition to the optically pure tert-butanesulfinyl aldimines under the optimized conditions.
中文翻译:

叔丁烷亚磺酰胺的立体选择性亲核单氟甲基化:外消旋α-氟碳负离子的动态热力学解析。
已开发出以α-氟代-α-苯硫基-α-苯基磺酰基甲烷(FTSM)为试剂的叔丁烷亚磺酰基醛亚胺的非对映选择性亲核单氟甲基化试剂,可提供良好产率的α-单氟甲基胺,且对单氟甲基化碳都有良好的立体控制和相邻的带有磺酰基的氟化碳。外消旋α-氟-α-苯硫基-α-苯基磺酰甲基阴离子由于在优化条件下向光学纯叔丁亚磺酰基亚胺中添加的可逆性而经历了动态热力学拆分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号