当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging.
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.biomaterials.2020.119763 Jian He 1 , Yue Qiao 1 , Hongbo Zhang 2 , Jun Zhao 3 , Wanli Li 4 , Tingting Xie 4 , Danni Zhong 4 , Qiaolin Wei 1 , Shiyuan Hua 1 , Yinhui Yu 5 , Ke Yao 5 , Hélder A Santos 6 , Min Zhou 7
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-08 , DOI: 10.1016/j.biomaterials.2020.119763 Jian He 1 , Yue Qiao 1 , Hongbo Zhang 2 , Jun Zhao 3 , Wanli Li 4 , Tingting Xie 4 , Danni Zhong 4 , Qiaolin Wei 1 , Shiyuan Hua 1 , Yinhui Yu 5 , Ke Yao 5 , Hélder A Santos 6 , Min Zhou 7
Affiliation
|
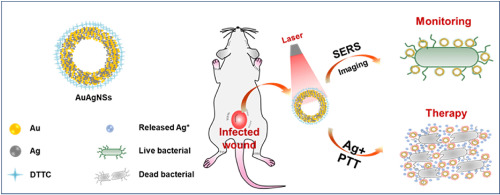
Chronic infections, caused by multidrug-resistant (MDR) bacteria, constitute a serious problem yet often underappreciated in clinical practice. The in situ monitoring of the bacteria-infected disease is also necessary to track and verify the therapeutic effect. Herein we present a facile approach to overcome the above challenges through a Raman tag 3,3'-diethylthiatricarbocyanine iodide (DTTC)-conjugated gold-silver nanoshells (AuAgNSs). With a strong responsive of the near-infrared laser due to surface plasmon resonance (SPR) from hybrid metallic nanoshell structure, AuAgNSs exhibits an efficient photothermal effect, and it simultaneously releases silver ions during laser irradiation to bacterial eradicate. Herein, two MDR bacteria strain, methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase Escherichia coli, are chosen as models and studied both in vitro and in vivo. As a result, the AuAgNSs-DTTC substrates enable surface-enhanced Raman scattering imaging to provide a non-invasive and extremely high sensitive detection (down to 300 CFU mL-1 for MRSA) and prolonged tracking (at least 8 days) of residual bacteria. In a chronic MRSA-infected wound mouse model, the AuAgNSs gel-mediated photothermal therapy/silver-release leads to a synergistic would healing with negligible toxicity or collateral damage to vital organs. These results suggest that AuAgNSs-DTTC is a promising anti-bacterial tool for clinical translation.
中文翻译:

金银纳米壳可促进耐药细菌感染的伤口愈合,并可通过表面增强拉曼散射成像进行监测。
由多药耐药性(MDR)细菌引起的慢性感染是一个严重的问题,但在临床实践中常常未被重视。对细菌感染的疾病进行原位监测对于追踪和验证治疗效果也是必要的。本文中,我们提出了一种通过拉曼标记3,3'-二乙基硫代三氢花青碘化物(DTTC)共轭的金银纳米壳(AuAgNSs)来克服上述挑战的简便方法。由于来自混合金属纳米壳结构的表面等离振子共振(SPR),AuAgNSs对近红外激光具有强响应性,因此AuAgNSs表现出有效的光热效应,并且在激光照射过程中同时释放银离子以消灭细菌。在此,我们介绍了两种耐多药细菌菌株:耐甲氧西林的金黄色葡萄球菌(MRSA)和广谱β-内酰胺酶大肠杆菌,选择作为模型并在体外和体内进行研究。结果,AuAgNSs-DTTC底物能够进行表面增强拉曼散射成像,从而提供无创且极高灵敏度的检测(对于MRSA,检测低至300 CFU mL-1),并能长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。AuAgNSs-DTTC底物可进行表面增强拉曼散射成像,从而提供无创且极高的灵敏度检测(对于MRSA,检测低至300 CFU mL-1),并可长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。AuAgNSs-DTTC底物可进行表面增强拉曼散射成像,从而提供无创且极高的灵敏度检测(对于MRSA,检测低至300 CFU mL-1),并可长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。
更新日期:2020-01-09
中文翻译:

金银纳米壳可促进耐药细菌感染的伤口愈合,并可通过表面增强拉曼散射成像进行监测。
由多药耐药性(MDR)细菌引起的慢性感染是一个严重的问题,但在临床实践中常常未被重视。对细菌感染的疾病进行原位监测对于追踪和验证治疗效果也是必要的。本文中,我们提出了一种通过拉曼标记3,3'-二乙基硫代三氢花青碘化物(DTTC)共轭的金银纳米壳(AuAgNSs)来克服上述挑战的简便方法。由于来自混合金属纳米壳结构的表面等离振子共振(SPR),AuAgNSs对近红外激光具有强响应性,因此AuAgNSs表现出有效的光热效应,并且在激光照射过程中同时释放银离子以消灭细菌。在此,我们介绍了两种耐多药细菌菌株:耐甲氧西林的金黄色葡萄球菌(MRSA)和广谱β-内酰胺酶大肠杆菌,选择作为模型并在体外和体内进行研究。结果,AuAgNSs-DTTC底物能够进行表面增强拉曼散射成像,从而提供无创且极高灵敏度的检测(对于MRSA,检测低至300 CFU mL-1),并能长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。AuAgNSs-DTTC底物可进行表面增强拉曼散射成像,从而提供无创且极高的灵敏度检测(对于MRSA,检测低至300 CFU mL-1),并可长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。AuAgNSs-DTTC底物可进行表面增强拉曼散射成像,从而提供无创且极高的灵敏度检测(对于MRSA,检测低至300 CFU mL-1),并可长期追踪(至少8天)残留细菌。在慢性MRSA感染的伤口小鼠模型中,AuAgNSs凝胶介导的光热疗法/银释放导致协同作用的愈合,对生命器官的毒性或副作用也可忽略不计。这些结果表明,AuAgNSs-DTTC是用于临床翻译的有前途的抗菌工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号